Science - Freezing | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Freezing
Freezing
Let us now try to reverse the process. Let us start
with the liquid and cool it slowly. What happens?

How does the temperature of a liquid vary when it is cooled till it freezes?
Now let us start with the liquid naphthalene that
we got from the previous experiment. Let us allow it to cool while observing
the temperatures at regular intervals of time till the liquid completely
freezes or solidifies. Let us plot a graph of temperature versus time. This
curve is called the cooling curve. This shows that how the temperature of a
pure solid changesasitiscooledtoitsfreezingpointand beyond.
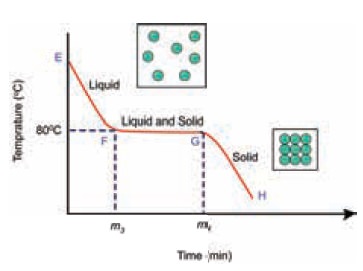
Cooling Curve
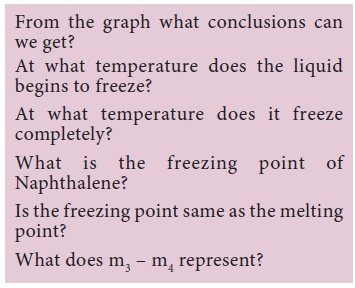
Let us now analyse the curve.

Why the temperature remains a constant between F – G?
The entire heat energy is given out at this stage
as the particles of the liquid get attracted to each other. This released
energy is absorbed by the surroundings.
Hence there is no increase in temperature for
naphthalene. Both liquid and solid states co- exist at this stage. This hidden
energy is called latent heat of freezing which is the same as latent heat of
fusion, This latent heat is released when there is a change of state from
liquid to solid.
Related Topics