Science - Types of Mixtures | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Types of Mixtures
Types of Mix tures
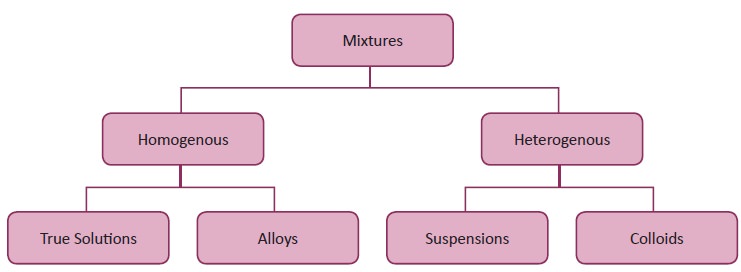
1. Homogenous and heterogeneous mix tures
Let us try to differentiate a homogenous mixture from a heterogeneous mixture
In a homogeneous mixture the components are
uniformly mixed and it will have single phase.
In heterogeneous mixture are not mixed thoroughly
or uniformly, and it will have more than single phase.
Mix some Iron filings and common salt in a glass
plate. Observe.

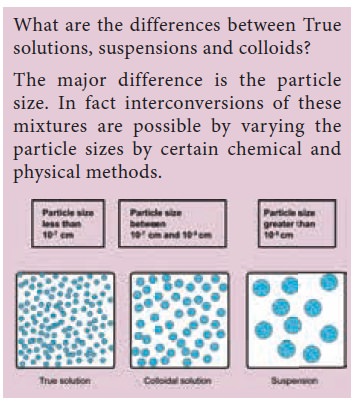
Let us now try to differentiate a true solution from colloidal solution and suspension.
Observations
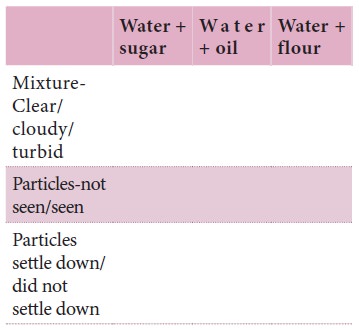
We can see that in the case of sugar we get a clear
solution and the particles never settle down. In the case of oil and water we
first get a cloudy mixture which separates into layers after a while. In the
case of flour mixed with water we get a very turbid mixture and fine particles
slowly settle down at the bottom after some time. We can call the first mixture
as homogeneous mixture and a true solution. The second one was apparently
homogeneous for a while but separated into layers, leaving behind some
cloudiness. This is called a colloidal solution. The third one is heterogeneous
and is called a suspension in which the particles settle down at the bottom.
2. Differences between homogenous and heterogeneous mix
tures
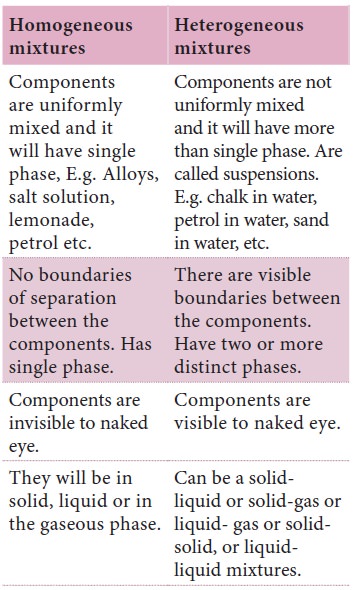
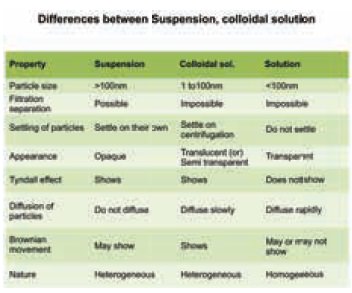
The following table summarises the differences between the three types of mixtures
Try this on your own
The longest wavelength of red light (almost
infrared) that most people can see is 7.5 × 10-7 meters. What is
this in nanometres?
Length in nm = (length in m) × (109
nm/m)
Related Topics