Matter Around Us | Science - Liquids | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Liquids
Liquids
1. Why do liquids not have shape?
According to the kinetic particle theory of matter
the particles in liquids.
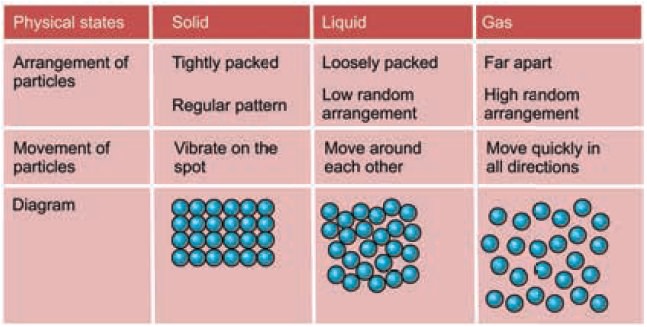
1. Are not
arranged in an orderly manner;
2. Are held
together by weak forces of attraction;
3. Have more
kinetic energy than the particles of solids;
4. Are free
to move throughout the medium by colliding over each other.
2. Why do liquids have fixed
volume?
The particles in liquids are slightly away from
each other compared to solids. They are packed quite closer to each other.
Moreover the forces of attraction between them help to stay together. Thus
liquids cannot be compressed and they have fixed volume.
Related Topics