Science - Boiling | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Boiling
Boiling
Boiling refers to the process by which a substance
changes from the liquid state to the gaseous state at its boiling point. Different
liquids have different boiling points.
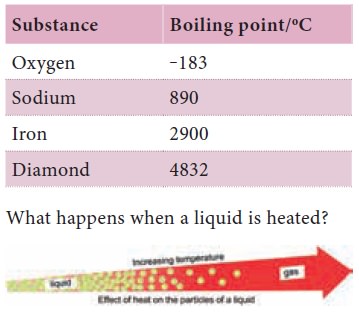
Boiling points of a few substances
Heating curves and cooling curves
A heating curve is a graph showing the temperature
of a substance plotted against the amount of energy it has absorbed. You may
also see a cooling curve, which is obtained when a substance cools down and
changes state.
How does the temperature of a liquid change when it is heated to its boiling point?
Let us take a liquid say water and heat it slowly
till it boils while observing the temperature at regular intervals of time. If
we plot a graph of temperature against time we will get one curve similar to
the one shown below.
Vaporisation curve of a liquid
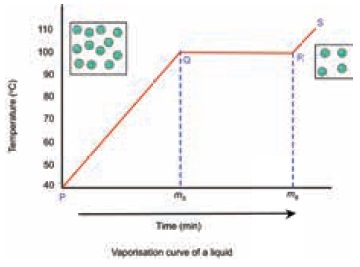
From the graph what conclusions can we get?

Let us now analyse the curve.

Why the temperature remains a constant between Q-R?
Entire heat energy is absorbed at this stage is
used to overcome the attractive forces between the liquid particles which are
intact. The particles start moving faster as their kinetic energy increases.
Hence there is no increase in temperature. This hidden energy is called latent
heat of vaporisation. The heat energy that is absorbed at this stage is
exclusively used for change of state from liquid to vapour.
The following curve sums up what we have been
discussed so far
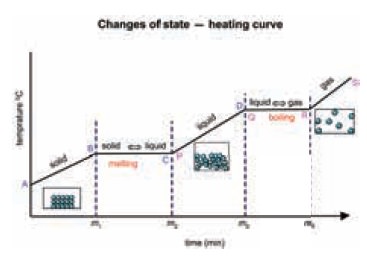
Heating Curve
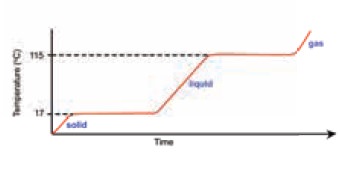
What is the melting point of this substance?
What is the boiling point of this substance?
What is the state of the substance at room
temperature (21oC)

Evaporation and Boiling

Related Topics