Science - Is Matter Particulate or Continuous | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Is Matter Particulate or Continuous
Is Matter Particulate or Continuous


Though in
1803 John Dalton proposed his atomic theory, no one could prove that matter
was made up of separate particles since they were too small to see. In 1827, a
Scottish botanist Robert Brown noticed, Pollen grains jiggling in water. He
used a microscope to look at pollen grains moving randomly in water. Initially
he thought that these pollen grains were to be some sort of unknown organisms.
He repeated the experiment with non-living substances like fine rock dust. To
his surprise he saw the same strange dance of the particles in the surface of
the water. They were non-living, but they were constantly moving, as if
something was making each of them to move. What could be there to make them
move? At this point, he could not explain why this occurred.
One possible explanation was that very small particles
in water were actually randomly moving all the time and were striking the
pollen particles from all sides, to make them move randomly. This erratic
movement of pollen grains later came to be known as Brownian motion.
Movement
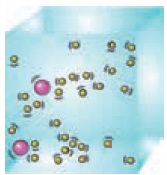

In 1905, physicist Albert Einstein explained that the pollen grains were being moved
by individual water particles or molecules. This confirmed that atoms and
molecules did exist, and provided evidence for particle theory as well as they
were on continuous motion. Particles in both liquids and gases (collectively
called fluids) move randomly. They do this because they were bombarded by the
other moving particles in the fluid. Larger particles can be moved by light,
fast-moving molecules. It was only in 1908, observations backed with
calculations had confirmed that atoms were real.
Today we
are very convinced that atoms and molecules are not mere speculations. Using
very sophisticated methods like
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM),
has actually made it possible to see atoms, like in the picture below.
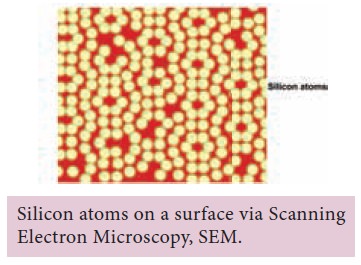
The atomic fact is: “All things are
made of atoms – tiny little particles moving around continuously, attracting
each other when they are a short distance apart, but repelling when they are
squeezed very close.”
Have you ever seen dust particles ‘dancing’ when a
narrow beam of light enters a dark room?
This is yet another example of Brownian motion. Air
is made of tiny particles that move around. These moving particles bump into
dust particles making them move irregularly or dance. Air particles are tiny to
be seen. Hence, we can see only dust particles.
These observations led to the kinetic particle
theory of matter. According to this theory all matter is made up of tiny
particles and these particles are in constant motion, which possesses kinetic
energy.
‘Kinetic’ means motion, based on this we are going
to describe the differences in the properties of solids, liquids and gases and
the changes in states of matter.
Related Topics