Science - Melting | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Melting
Melting
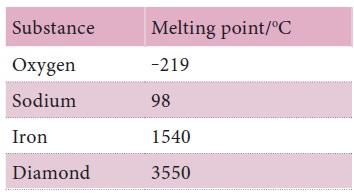
A substance absorbs heat energy and it melts. The
temperature at which a substance melts is called as melting point. Different
substances have different melting points. Hard substance such as diamond also
melts.
Melting points of a few substances
1. What happens when a solid is heated until it melts?

How temperature of a solid does varies on heating?
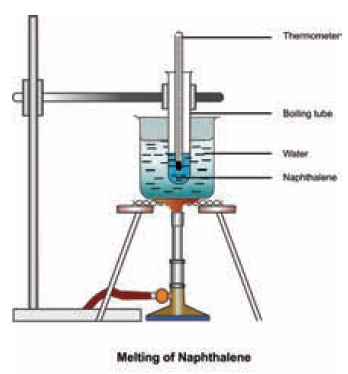
Melting point apparatus set up is as shown below.
We can study the melting of solid naphthalene by varying the temperature with
time.
Let us observe the variation of temperature of the
solid while it is heated at regular intervals of time. We can continue heating
till entire solid melts and a little beyond. If we plot a graph of temperature
versus time, we get a melting curve as shown below.
Melting Curve
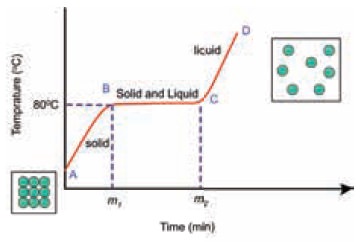
From the graph what conclusions can we get?
Let us try to answer the following questions.

Let us now analyse the curve.
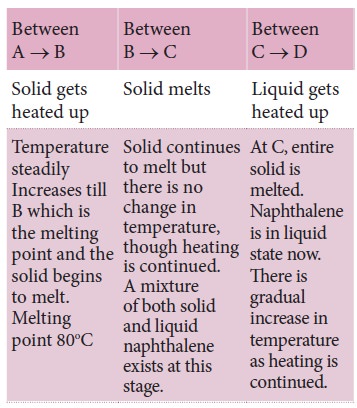
Why the temperature remains constant between B – C?
The entire heat energy absorbed is used to overcome
the attractive forces between the solid particles, which are held in xed
positions. Hence, there is no increase in temperature. is hidden energy is
called latent heat of fusion, which is exclusively used for change of state
from solid to liquid.
Related Topics