Chapter: 9th Science : Matter Around Us
Changes in States of Matter and the Kinetic Particle Theory
Changes in States of Matter and the Kinetic Particle Theory
Change of state
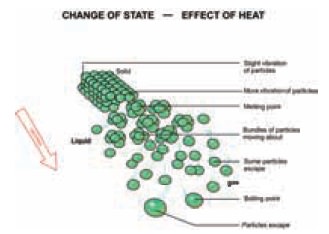
Matter can change from one state to another. When
you taste an ice cream it changes from solid to liquid state due to the
transfer of heat energy from your body to the ice cream. According to kinetic
particle theory, particles of matter are in constant motion as they possess
kinetic energy. As we discussed earlier, gases have more kinetic energy than
the liquids and solids. Solids have the least kinetic energy.
When matter is either heated or cooled, heat energy
is either absorbed or given out. is causes change in the energy of the
particles leading to change of state. These changes are reversible physical
changes.
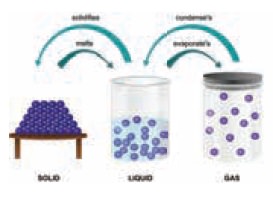
Changes of states
·
solid melts into liquid
·
liquid vaporises into gas
·
gas condenses into liquids
·
liquid freezes or solidifies into solid

Related Topics