Science - Compound - Matter Around Us | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Compound - Matter Around Us
Compound
A compound is made of two or more of elements
combined in a fixed ratio by mass. For example water is made up of two elements,
hydrogen and oxygen. Similarly, cane sugar is made up of three elements carbon,
hydrogen and oxygen. A compound has a definite formula. Examples - water is H2O,
cane sugar is C12H22O11.
The properties of a compound are entirely different
from their constituent elements. For e.g. Iron Sulphide does not show the
properties of neither sulphur nor iron. Try waving a magnet over Iron Sulphide?
Does it get attracted to the magnet? No.
Compare and Contrast
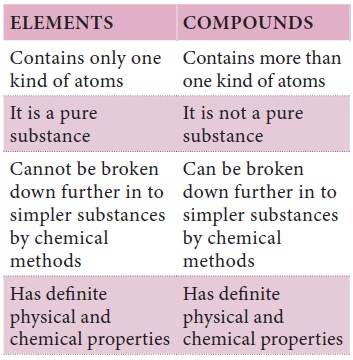
We can classify matter as pure and impure substances

Mixtures contain more than one substances. These
are made by physically mixing two or more elements or compounds in any random
proportion by mass or volume. For example Gunpowder is a mixture of sulphur,
potassium nitrate and charcoal. Here individually each component by itself is a
pure substance.
You will be able to find several examples of
mixtures that we come across and use in our daily life.

Do it yourself: Collect various labels of food
products, medicines, juices, etc. and discuss the ingredients present in them
and tabulate it.
Let us see the differences between mixtures and
compounds.
Related Topics