Matter Around Us | Science - Gases | 9th Science : Matter Around Us
Chapter: 9th Science : Matter Around Us
Gases
Gases
1. Why do gases not have fixed shape?
According to the kinetic theory of matter the
particles in gases
1. Are not
close to each other but are spread far apart from each other;
2. Are not
held in any fixed positions;
3. Have very
weak forces of attraction between each other, lesser than liquids;
4. Have a
lot of kinetic energy and can move freely in all directions.
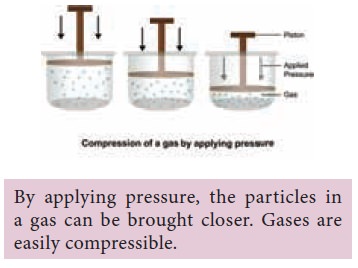
2. Why do gases not have fixed volume?
Since the particles in gases are far apart there is
a lot of space between them. Therefore, they can be forced to get closer or in
other words can easily be compressed.
Light, sound, heat etc. are not matter. They are
different forms of Energy.
Related Topics