Introduction - Atomic Structure | 9th Science : Atomic Structure
Chapter: 9th Science : Atomic Structure
Atomic Structure
Atomic Structure
Introduction
We already know that anything that has definite
mass and occupies space is known as matter.
Let us quickly recall
What is matter?
What are the different states of matter?
Is matter continuous or particulate in nature?
If somehow we could go on dividing any piece of
matter we will get smaller and smaller particles until we reach the smallest
particle of it which cannot be divided further. These smallest particles can be
atoms, molecules or ions.
Atoms are the building blocks of matter. Every substance
is made up of atoms in one form or other. Different kinds of atoms have
different properties (both physical and chemical).
You already know that atoms combine together to
form molecules. This combination is called a chemical reaction which can be
represented symbolically by balanced chemical equations.
Now look at the following equation. What do you
understand?
We can say that Sodium and Chlorine combine to form
Sodium Chloride.
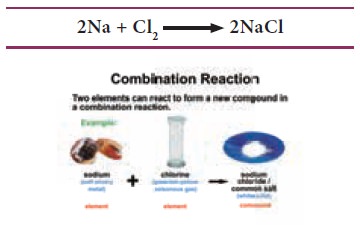
What is a combination reaction?
Combination reaction is a reaction where two or
more substances combine to form a single substance. The combination of
different elements to form a compound is governed by certain basic rules. These
rules are known as Laws of chemical combination.
Related Topics