Rutherford’ s model, Bohr’ s model - Discovery of Nucleus | 9th Science : Atomic Structure
Chapter: 9th Science : Atomic Structure
Discovery of Nucleus
Discovery of Nucleus
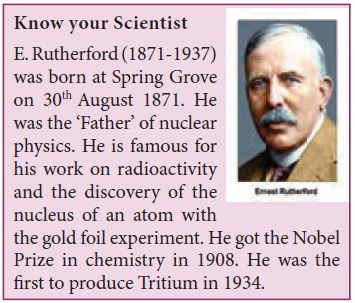
Thomson’s model of atoms is a conceptual
representation like many other models in science. Scientists test scientific
models by doing experiments to find out if they were wrong. The model proposed
by Thomson was conceptual. Scientists were eager to test it by doing an
experiment. How would you test if the model is correct or wrong? They are so
small that even a powerful microscope is useless in peering inside an atom.
In 1905, Ernest Rutherford along with his scholars
Hans Geiger and Ernest Marsden came up with an interesting idea to test the
omson’s model. In omson’s model recall that the charges are symmetrically
distributed. Suppose you shoot a highly energetic positively charged particle
smaller than an atom, to collide at an atom, what do you expect? As the
incoming particle is positive, it should be repelled by the positive atom. is
is because you know that Àùlike charges repel each other.Àù If according to plum
pudding model, the positive charge of atoms is evenly distributed; it should be
very small at each point inside the atom. But as the energy of the incoming
particle is higher than the repulsion at the point of contact, the particle
should overcome the repulsion and penetrate the atom.
Once it is inside the atom, the positively charged
particle is repulsed on all sides with the same force. Assuming that atom is a
uniformly positively charged mass with random moving electrons, the particle
should come out of the other end of the atom almost unde ected. Some of the
electrons inside the atom could attract the positively charged particle and
make small change in the path. Therefore it can be predicted that deviation if
any, be less than a small fraction of a degree and is negligible.
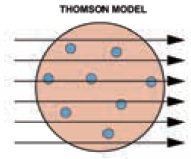
1. Rutherford’ s – ray scattering ex periment
Alpha particles are positively charged it possess
adequate energy to overcome the repulsive force of positive charge, if the
charge is evenly distributed in an atom. As you probably know, according to
Coulomb’s law, the less concentrated a sphere of electric charge is, the weaker
is its electric field at its surface.
Atoms are so small that you cannot pick them one by
one to be kept as a target and shoot alpha particles. Gold as you may know is a
highly malleable metal and can be made in to a very thin layer.
They arranged an experimental set up. A natural
radioactive source that emitted highly energetic alpha particles was chosen.
The source was kept inside a lead box with a small hole in it. Alpha particles
came out of the source in all directions. Those particles which hit the walls
of the box were absorbed by it. Only those alpha particles that were emitted in
the direction of the hole could escape. These rays of alpha particles followed
a straight line.
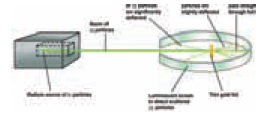
A thin gold foil, about 400 atoms thick, was kept
on the path of the alpha particle. They also kept a circular screen coated with
zinc sulphide surrounding the foil. When an alpha particle hit the screen, it
would produce uorescence glow in the point where they struck the screen. From
the point on the screen, one can infer the path taken by the alpha particle after
penetrating the gold foil. The whole set up was kept inside a vacuum glass
chamber, to avoid alpha particles from interacting and getting scattered by air
molecules.
The experiments were repeated for reproducibility.
Each time when the experiment was conducted, they computed and tabulated the
angle of the rays of alpha particle after it hits the gold foil. They observed
the following.
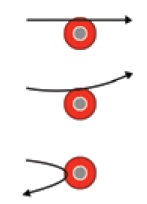
(I) Most of the fast moving α-particles passed
straight through the gold foil.
(II) Some α particles were deflected by small
angles and a few by large angles.
(III) Surprisingly very few α particles completely
rebounded.
The experiments showed that most of the alpha
particles behaved as expected, but there was a small discrepancy. Out of every
2000 particles that got scattered, just one was de ected by a full 180°. at is,
they simply retraced their path after hitting the gold foil. You know that
change of direction is possible only if a strong enough force acted against the
direction of the motion of the particle.
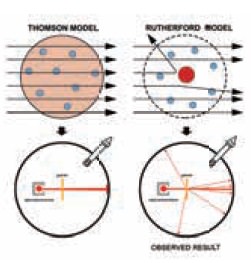
Based on the plum pudding model of the atom, it was
assumed that there was nothing dense or heavy enough inside the gold atoms to
de ect the massive alpha particles from their paths. However, what Rutherford
actually observed did not match his prediction. These observations indicated
that a new model is needed to account for the evidences gathered in the
experiment.
Rebound of alpha particle was impossible under the
Thomson model. The alpha particle could have been deflected at 180° only if the
positive charge was concentrated at a point rather than dispersed throughout
the atom. If all the positive charge of the atom was concentrated at a small
area inside the atom, only then, the electrostatic repulsion would be strong
enough to bounce them back at 180°.
Now two observational evidence were before
Rutherford and his team
1) Most of
the particles passed are not deviated as there was no obstruction to their
path: This should imply that most part of the atom is empty
2) Some
alpha particle was deflected right back; implying that the positive charge
should be concentrated at the centre of atom.
To be sure that their findings were really correct,
the team performed the same type of experiments with many other materials
including gases between the period 1908 and 1913.
Based upon these evidences, Rutherford rejected the
omson’s idea and proposed that all the positive charges are concentrated in the
central region of the atom called ‘nucleus’, and electrons orbit the nucleus at
a distance. Further he stated that in between the nucleus and electron inside
an atom there existed a void. is came to be called as planetary model of atom.
2. Rutherford’ s model of an atom- salient features
i.
Atom has a very small nucleus at the centre.
ii.
There is large empty space around the nucleus.
iii.
Entire mass of an atom is concentrated in a very
small positively charged region which is called the nucleus.
iv.
Electrons are distributed in the vacant space around
the nucleus.
v.
The electrons move in circular paths around the
nucleus.
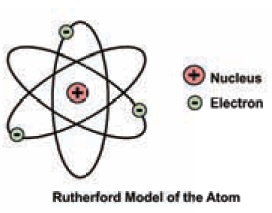
3. Limitations in Rutherford’ s model
Although the model suggested by Rutherford went
beyond the one by omson and explained the behaviour of alpha particles, it also
le a few questions unanswered. Planets can go around the Sun under the
gravitational attraction. But negatively charged electron should be attracted
by the positively charged nucleus, since opposite charges attract. But it does
not happen that way.
It was shown by Clark Maxwell that a charged body
moving under the in uence of attractive force loses energy continuously in the
form of electromagnetic radiation. us unlike a planet the electron is a charged
body and it emits radiations while revolving around the nucleus. As a result,
the electron should lose energy at every turn and move closer and closer to the
nucleus following a spiral path consequently the orbit will become smaller and
smaller and nally the electron will fall into the nucleus. In other words, the
atom should collapse. However, this never happens and atoms are stable.
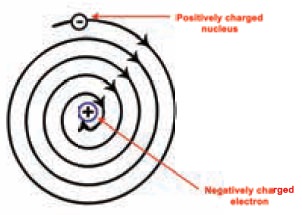
Thus the stability of the atom could not be
explained by Rutherford Model. There were also a few more objections to his
model. This led on to more research and evolving better models of atomic
structure.
4. Bohr’ s model of an atom

A new model of atom was needed because Rutherford
model could not explain the stability of atom. Neils Bohr developed a
successful model of hydrogen atom. In order to justify the stability of an atom
Neils Bohr made some improvements on Rutherford’s model. The main postulates
are:
I.
In atoms, electrons revolve around the nucleus in
certain special or permissible orbits known as discrete orbits or shells or energy
levels
II.
While revolving in these discrete orbits the
electrons do not radiate energy.
III.
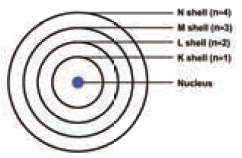
The circular orbits are numbered as 1, 2, 3, 4,… or
designated as K, L, M, N, shells. These numbers are referred to as principal
quantum numbers (n).
IV.
K shell (n=1) is closest to the nucleus and is
associated with lowest energy. L, M, N, …. etc are the next higher energy
levels. As the distance from the nucleus increases the energy of the shells
also increases.
V.
The energy of each orbit or shell is a xed quantity
and the energy is quantized.
VI.
As the distance from the nucleus increases, the
size of the orbits also increases.
VII.
Maximum number of electrons that can be
accommodated in an energy level is given by 2n2 where n is the principal
quantum number of the orbit.
VIII.
When an electron absorbs energy, it jumps from lower energy level to
higher energy level.
IX.
When an electron returns from higher energy level to lower energy level, it
gives o energy.
5. Limitations of Bohr’ s model
Many arguments were raised against Bohr’s model of
an atom. One main limitation was that his model was applicable only to
Hydrogen. It could not be extended to multi electron atoms. Hence more research
and deeper study of atoms became necessary. A detailed study of these aspects
will be done in higher classes.
Orbit or shell:
Orbit is defined as the path by which electrons
revolve around the nucleus.
Illustration:
The number of electrons in the first orbit (K)(n =
1);2 x 12 = 2
The number of electrons in the second orbit (L) (n
= 2); 2 x 22 = 8
Related Topics