Chapter: 11th Chemistry : UNIT 2 : Quantum Mechanical Model of Atom
Hund's rule of maximum multiplicity
Hund's rule of maximum multiplicity
The Aufbau principle describes how the electrons are filled in various orbitals. But the rule does not deal with the filling of electrons in the degenerate orbitals (i.e. orbitals having same energy) such as px, py and pz. In what order these orbitals to be filled? The answer is provided by the Hund's rule of maximum multiplicity. It states that electron pairing in the degenerate orbitals does not take place until all the available orbitals contains one electron each.
We know that there are three p orbitals, five d orbitals and seven f orbitals. According to this rule, pairing of electrons in these orbitals starts only when the 4th, 6th and 8th electron enters the p, d and f orbitals respectively.
For example, consider the carbon atom which has six electrons. According to Aufbau principle, the electronic configuration is 1s2, 2s2, 2p2
It can be represented as below,
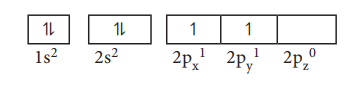
In this case, in order to minimise the electron-electron repulsion, the sixth electron enters the unoccupied 2py orbital as per Hunds rule. i.e. it does not get paired with the fifth electron already present in the 2Px orbital.
Related Topics