Chapter: Modern Pharmacology with Clinical Applications: Pharmacological Management of Chronic Heart Failure
Disopyramide
Disopyramide
Disopyramide (Norpace) can suppress atrial and
ven-tricular arrhythmias and is longer acting than other drugs in its class.
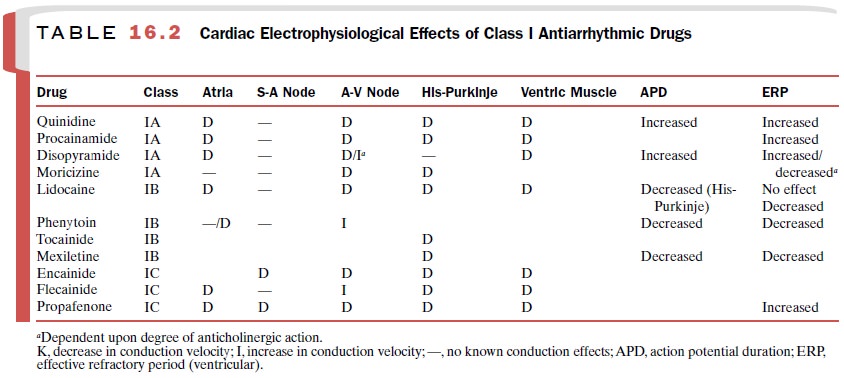
Electrophysiological Actions
The effects of disopyramide
on the myocardium and specialized conduction tissue (Table 16.2) are a
com-posite of its direct actions on cardiac tissue and its indi-rect actions
mediated by competitive blockade of mus-carinic cholinergic receptors.
Sinoatrial Node
The direct depressant actions
of disopyramide on the sinoatrial node are antagonized by its anticholiner-gic
properties, so that at therapeutic plasma concentra-tions, either no change or
a slight increase in sinus heart rate is observed. Both the anticholinergic and
direct de-pressant actions of disopyramide on sinus automaticity appear to be
greater than those of quinidine.
Atrium
Disopyramide reduces membrane
responsiveness in atrial muscle and the amplitude of the action potential.
Excitability of atrial muscle is decreased. These changes decrease atrial
muscle conduction velocity. Action po-tential duration in atrial muscle fibers
is prolonged by disopyramide administration. This occurrence increases ERP.
Postrepolarization refractoriness does not occur with disopyramide, and it appears
to differ from quini-dine and procainamide in this respect.
Abnormal atrial automaticity
may be abolished at disopyramide plasma concentrations that fail to alter
ei-ther conduction velocity or refractoriness. Disopyramide increases atrial
refractoriness in patients pretreated with atropine, suggesting that the
primary action of disopyra-mide is a direct one and not a consequence of its
anti-cholinergic effect.
A-V Node
Disopyramide depresses
conduction velocity and in-creases the ERP of the A-V node through a direct
ac-tion. Its anticholinergic actions, however, produce an in-crease in
conduction velocity and a decrease in the ERP. The net effect of disopyramide
on A-V nodal transmission therefore will be determined by the sum of its direct
depression and indirect facilitation of trans-mission.
His-Purkinje System and Ventricular Muscle
Disopyramide administration
reduces membrane responsiveness in Purkinje fibers and ventricular mus-cle and
reduces the action potential amplitude. Even greater depression may occur in
damaged or injured myocardial cells. Action potentials are prolonged after
disopyramide administration, and this results in an in-crease in the ERPs of
His-Purkinje and ventricular muscle tissue. Unlike procainamide and quinidine,
disopyramide does not produce postrepolarization re-fractoriness.
The effect of disopyramide on
conduction velocity depends on extracellular K+ concentrations.
Hypo-kalemic patients may respond poorly to the antiar-rhythmic action of
disopyramide, whereas hyper-kalemia may accentuate the drug’s depressant
actions.
Electrocardiographic Changes
The electrocardiographic
changes observed after disopyramide administration are identical to those seen
with quinidine and procainamide.
Hemodynamic Effects
Disopyramide directly
depresses myocardial contractil-ity. The negative inotropic effect may be
detrimental in patients with compromised cardiac function. Some pa-tients
develop overt congestive heart failure. At usual therapeutic doses, depression
of myocardial function is not a problem in most patients with normal
ventricular function.
Despite the decrease in
cardiac output produced by disopyramide, blood pressure is well maintained by a
reflex increase in vascular resistance. Catecholamine administration can reverse
the myocardial depression.
Pharmacokinetics
The salient pharmacokinetic
features of disopyramide:
Oral bioavailability : 87–95%
Onset of action : 30
minutes–3.5 hours
Peak response : 30 minutes–3
hours
Duration of action : 1.5–8.5
hours
Plasma half-life : 4–10 hours
Primary route of metabolism: Hepatic,
active metabolite
Primary route of : 80% renal
(50% unchanged);
excretion : 15% biliary
Therapeutic serum Concentration
: 1–5 μg /mL
Clinical Uses
The indications for use of
disopyramide are similar to those for quinidine, except that it is not approved
for use in the prophylaxis of atrial flutter or atrial fibrilla-tion after DC
conversion. The indications are as follows: unifocal premature (ectopic)
ventricular contractions, premature (ectopic) ventricular contractions of
multifo-cal origin, paired premature ventricular contractions (couplets), and
episodes of ventricular tachycardia. Persistent ventricular tachycardia is
usually treated with DC conversion.
Adverse Effects
The major toxic reactions to
disopyramide administra-tion include hypotension, congestive heart failure, and
conduction disturbances. These effects are the result of disopyramide’s ability
to depress myocardial contractil-ity and myocardial conduction. Although
disopyramide initially may produce ventricular tachyarrhythmias or ventricular
fibrillation in some patients, the incidence of disopyramide-induced syncope in
long-term therapy is not known. Most other toxic reactions (e.g., dry mouth,
blurred vision, constipation) can be attributed to the an-ticholinergic
properties of the drug.
CNS stimulation and
hallucinations are rare. The in-cidence of severe adverse effects in long-term
therapy may be lower than those observed with quinidine or procainamide.
Contraindications
Disopyramide should not be
administered in cardio-genic shock, preexisting second- or third-degree A-V
block, or known hypersensitivity to the drug. Neither should it be given to
patients who are poorly compen-sated or those with uncompensated heart failure
or se-vere hypotension. Because of its ability to slow cardiac conduction,
disopyramide is not indicated for the treat-ment of digitalis-induced
ventricular arrhythmias. Patients with congenital prolongation of the QT
interval should not receive quinidine, procainamide, or disopyra-mide because
further prolongation of the QT interval may increase the incidence of
ventricular fibrillation.
Because of its
anticholinergic properties, disopyra-mide should not be used in patients with
glaucoma. Urinary retention and benign prostatic hypertrophy are also relative
contraindications to disopyramide therapy. Patients with myasthenia gravis may
have a myasthenic crisis after disopyramide administration as a result of the
drug’s local anesthetic action at the neuromuscular junction. The elderly
patient may exhibit increased sen-sitivity to the anticholinergic actions of
disopyramide.
Caution is advised when
disopyramide is used in conjunction with other cardiac depressant drugs, such
asverapamil, which may adversely affect atrioventricular conduction.
Drug Interactions
In the presence of phenytoin,
the metabolism of disopy-ramide is increased (reducing its effective
concentra-tion) and the accumulation of its metabolites is also increased,
thereby increasing the probability of anti-cholinergic adverse effects.
Rifampin also stimulates the hepatic metabolism of disopyramide, reducing its
plasma concentration.
Unlike quinidine,
disopyramide does not increase the plasma concentration of digoxin in patients
receiv-ing a maintenance dose of the cardiac glycoside. Hypoglycemia has been
reported with the use of disopyramide, particularly in conjunction with
moder-ate or excessive alcohol intake.
Related Topics