Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Pharmacokinetics and Pharmacodynamics of Peptide and Protein Drugs
Chemical Modifications for Optimizing the Pharmacokinetics of Protein Therapeutics - Pharmacokinetics of Protein Therapeutics
Chemical Modifications for Optimizing the Pharmacokinetics
of Protein Therapeutics
In recent years, approaches modifying the molecular structure of protein
therapeutics have repeatedly been applied to affect the immunogenicity,
pharmacoki-netics, and/or pharmacodynamics of protein drugs. These approaches
include the addition, deletion or exchange of selected amino acids within the
protein’s sequence, synthesis of truncated proteins with a reduced amino acid
sequence, glycosylation or degly-cosylation, and covalent linkage to polymers
(Veronese and Caliceti, 2006). The latter approach has been used for several
protein therapeutics by linking them to monomethoxy polyethylene glycol (PEG)
molecules of various chain lengths in a process called PEGylation (Caliceti and
Veronese, 2003).
The conjugation of high polymeric mass to protein drugs is generally
aimed at preventing the protein from being recognized by the immune system as
well as reducing its elimination via glomerular filtration or proteolytic
enzymes, thereby prolonging the oftentimes relatively short elimina-tion
half-life of endogenous proteins. Conjugation of protein drugs with PEG chains
increases their molecular weight, but because of the attraction of water
molecules by PEG even more their hydro-dynamic volume, which in turn results in
a reduced renal clearance and restricted volume of distribution. PEGylation can
also shield antigenic determinants on the protein drug from detection by the
immune system through steric hindrance (Walsh et al., 2003). Similarly, amino
acid sequences sensitive towards proteolytic degradation may be shielded
against protease attack. By adding a large, hydrophilicmolecule to the protein,
PEGylation can also increase drug solubility (Molineux, 2003).
PEGylation has been used to improve the therapeutic properties of
numerous protein therapeu-tics including interferon-a, asparaginase, and filgras-tim. More details on the general concept of
PEGylation and its specific application for protein therapeutics.
The therapeutic application of L-asparaginase in the treatment of acute
lymphoblastic leukemia has been hampered by its strong immunogenicity with
allergic reactions occurring in 33% to 75% of treated patients in various
studies. The development of pegaspargase, a PEGylated form of L-asparaginase, is
a successful example for overcoming this high rate of allergic reactions
towards L-asparaginase using PEG conjugation techniques (Graham, 2003).
Pegaspargase is well tolerated compared to L-asparaginase, with 3% to 10% of
the treated patients experiencing clinical allergic reactions.
Pegfilgrastim is the PEGylated version of the
granulocyte-colony-stimulating factor filgrastim, which is administered for the
management of che-motherapy-induced neutropenia. PEGylation mini-mizes
filgrastim’s renal clearance by glomerular filtration, thereby making
neutrophil-mediated clear-ance the predominant route of elimination. Thus,
PEGylation of filgrastim results in so-called “self-regulating
pharmacokinetics” since pegfilgrastim has a reduced clearance and thus prolonged
half-life and more sustained duration of action in a neutropenic compared to a
normal patient because only few mature neutrophils are available to mediate its
elimination (Zamboni, 2003).
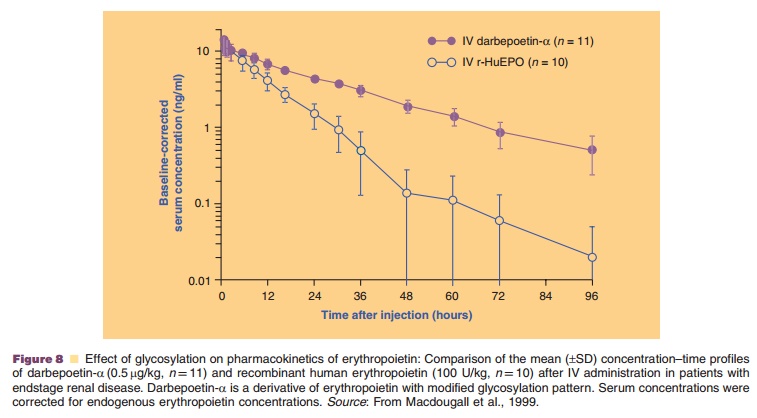
The hematopoietic growth factor darbepoetin-a is an example of a chemically modified endogenous protein with altered
glycosylation pattern. It is aglycosylation analog of human erythropoietin,
with two additional N-linked oligosaccharide chains (five in total) (Mould et
al., 1999). The additional N-glycosylation sites were made available through
substitution of five amino acid residues in the peptide backbone of
erythropoietin, thereby increasing the molecular weight from 30 to 37 kDa.
Darbepoetin-a has a substantially modified pharmacokinetic profile compared to
erythropoietin, resulting in a three-fold longer serum half-life that allows
for reduced dosing frequency (Fig. 8) (Macdougall et al., 1999). More details
on hematopoietic growth factors, including erythropoietin and darbepoetin-α, are provided.
Related Topics