Chapter: Basic & Clinical Pharmacology : Drug Receptors & Pharmacodynamics
Well-Established Second Messengers
Well-Established Second
Messengers
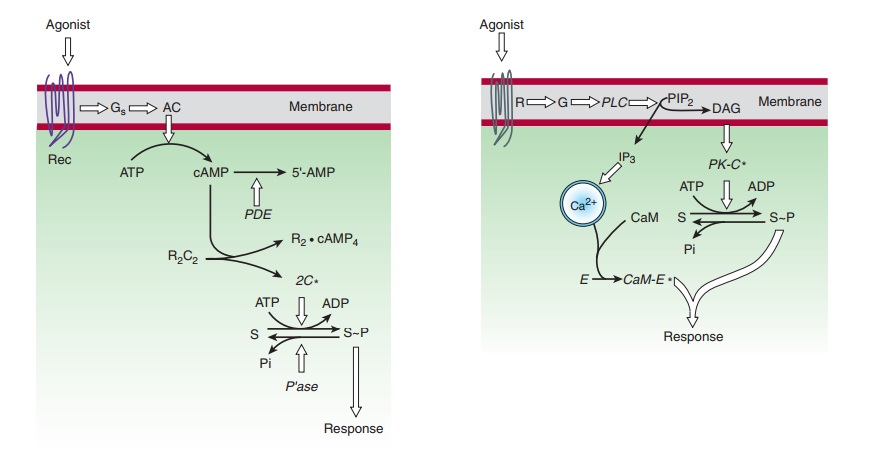
A. Cyclic Adenosine Monophosphate (cAMP)
Acting
as an intracellular second messenger, cAMP mediates such hormonal responses as
the mobilization of stored energy (the breakdown of carbohydrates in liver or
triglycerides in fat cells stimulated by β-adrenomimetic catecholamines), conservation
of water by the kidney (mediated by vasopressin), Ca2+ homeostasis
(regulated by parathyroid hormone), and increased rate and con-tractile force
of heart muscle (β-adrenomimetic
catecholamines). It also regulates the production of adrenal and sex steroids
(in response to corticotropin or follicle-stimulating hormone), relax-ation of
smooth muscle, and many other endocrine and neural processes.
cAMP
exerts most of its effects by stimulating cAMP-dependent protein kinases
(Figure 2–13). These kinases are composed of a cAMP-binding regulatory (R)
dimer and two catalytic (C) chains. When cAMP binds to the R dimer, active C
chains are released to diffuse through the cytoplasm and nucleus, where they
transfer phosphate from ATP to appropriate substrate proteins, often enzymes.
The specificity of the regulatory effects of cAMP resides in the distinct
protein substrates of the kinases that are expressed in different cells. For
example, liver is rich in phosphorylase kinase and glycogen synthase, enzymes
whose reciprocal regulation by cAMP-dependent phosphorylation governs
carbohydrate storage and release.
When the hormonal stimulus stops, the intracellular actions of cAMP are terminated by an elaborate series of enzymes. cAMP-stimulated phosphorylation of enzyme substrates is rapidly reversed by a diverse group of specific and nonspecific phos-phatases. cAMP itself is degraded to 5′-AMP by several cyclic nucleotide phosphodiesterases (PDE; Figure 2–13). Milrinone, a selective inhibitor of type 3 phosphodiesterases that are expressed in cardiac muscle cells, has been used as an adjunctive agent in treating acute heart failure. Competitive inhibition of cAMP deg-radation is one way that caffeine, theophylline, and other meth-ylxanthines produce their effects .
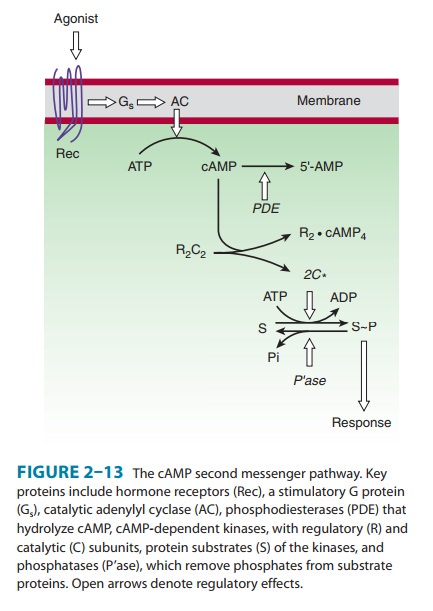
B. Phosphoinositides and Calcium
Another
well-studied second messenger system involves hormonal stimulation of
phosphoinositide hydrolysis (Figure 2–14). Some of the hormones,
neurotransmitters, and growth factors that trig-ger this pathway bind to
receptors linked to G proteins, whereas others bind to receptor tyrosine
kinases. In all cases, the crucial step is stimulation of a membrane enzyme,
phospholipase C (PLC), which splits a minor phospholipid component of the
plasma membrane, phosphatidylinositol-4,5-bisphosphate (PIP2), into
two second messengers, diacylglycerol
(DAG) and inositol-1,4,5-trisphosphate
(IP3or InsP3). Diacylglycerol is confined tothe
membrane, where it activates a phospholipid- and calcium-sensitive protein
kinase called protein kinase C. IP3 is water-solu-ble and diffuses
through the cytoplasm to trigger release of Ca2+ by binding to
ligand-gated calcium channels in the limiting mem-branes of internal storage
vesicles. Elevated cytoplasmic Ca2+ concentration
resulting from IP3-promoted opening of these channels promotes the
binding of Ca2+ to the calcium-binding protein calmodulin, which regulates
activities of other enzymes, including calcium-dependent protein kinases.
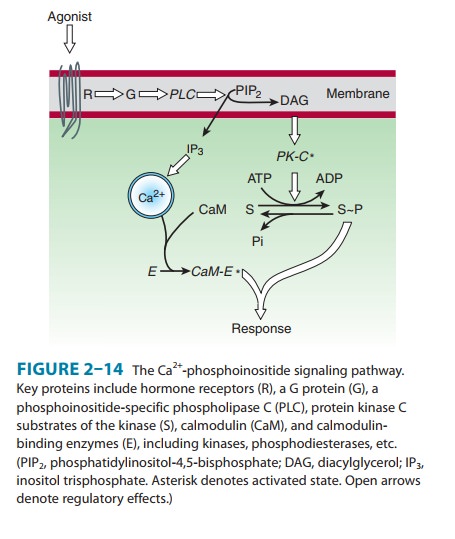
With
its multiple second messengers and protein kinases, the phosphoinositide
signaling pathway is much more complex than the cAMP pathway. For example,
different cell types may con-tain one or more specialized calcium- and
calmodulin-dependent kinases with limited substrate specificity (eg, myosin
light-chain kinase) in addition to a general calcium- and calmodulin-dependent
kinase that can phosphorylate a wide variety of protein substrates.
Furthermore, at least nine structurally distinct types of protein kinase C have
been identified.
As
in the cAMP system, multiple mechanisms damp or termi-nate signaling by this
pathway. IP3 is inactivated by dephosphory-lation; diacylglycerol is
either phosphorylated to yield phosphatidic acid, which is then converted back
into phospholipids, or it is deacylated to yield arachidonic acid; Ca2+ is actively removed
from the cytoplasm by Ca2+ pumps.
These
and other nonreceptor elements of the calcium-phos-phoinositide signaling
pathway are of considerable importance in pharmacotherapy. For example, lithium
ion, used in treatment of bipolar (manic-depressive) disorder, affects the
cellular metabo-lism of phosphoinositides .
C. Cyclic Guanosine Monophosphate (cGMP)
Unlike
cAMP, the ubiquitous and versatile carrier of diverse mes-sages, cGMP has
established signaling roles in only a few cell types. In intestinal mucosa and
vascular smooth muscle, the cGMP-based signal transduction mechanism closely
parallels the cAMP-mediated signaling mechanism. Ligands detected by
cell-surface receptors stimulate membrane-bound guanylyl cyclase to produce
cGMP, and cGMP acts by stimulating a cGMP-dependent protein kinase. The actions
of cGMP in these cells are terminated by enzymatic degradation of the cyclic
nucleotide and by dephos-phorylation of kinase substrates.
Increased
cGMP concentration causes relaxation of vascular smooth muscle by a
kinase-mediated mechanism that results in dephosphorylation of myosin light
chains (see Figure 12–2). In these smooth muscle cells, cGMP synthesis can be
elevated by two transmembrane signaling mechanisms utilizing two different
gua-nylyl cyclases. Atrial natriuretic peptide, a blood-borne peptide hormone,
stimulates a transmembrane receptor by binding to its extracellular domain,
thereby activating the guanylyl cyclase activ-ity that resides in the
receptor’s intracellular domain. The other mechanism mediates responses to
nitric oxide (NO;), which is generated in vascular endothelial cells in
response to natural vasodilator agents such as acetylcholine and histamine.
After entering the target cell, nitric oxide binds to and activates a
cytoplasmic guanylyl cyclase (see Figure 19–2). A number of use-ful
vasodilating drugs, such as nitroglycerin and sodium nitroprus-side used in
treating cardiac ischemia and acute hypertension, act by generating or
mimicking nitric oxide. Other drugs produce vasodilation by inhibiting specific
phosphodiesterases, thereby interfering with the metabolic breakdown of cGMP.
One such drug is sildenafil, used in treating erectile dysfunction and
pulmo-nary hypertension .
Related Topics