Chapter: Basic & Clinical Pharmacology : Drug Receptors & Pharmacodynamics
G Proteins & Second Messengers
G Proteins & Second
Messengers
Many
extracellular ligands act by increasing the intracellular con-centrations of
second messengers such as cyclic
adenosine-3′,5′-monophosphate
(cAMP), calcium ion, or the phosphoinositides (described
below). In most cases, they use a transmembrane sig-naling system with three
separate components. First, the extracel-lular ligand is selectively detected
by a cell-surface receptor. The receptor in turn triggers the activation of a G
protein located on the cytoplasmic face of the plasma membrane. The activated G
protein then changes the activity of an effector element, usually an enzyme or
ion channel. This element then changes the concentra-tion of the intracellular
second messenger. For cAMP, the effector enzyme is adenylyl cyclase, a membrane
protein that converts intracellular adenosine triphosphate (ATP) to cAMP. The
corre-sponding G protein, Gs, stimulates adenylyl cyclase after
being activated by hormones and neurotransmitters that act via specific Gs-coupled
receptors. There are many examples of such receptors, including β adrenoceptors,
glucagon receptors, thyrotropin recep-tors, and certain subtypes of dopamine
and serotonin receptors.

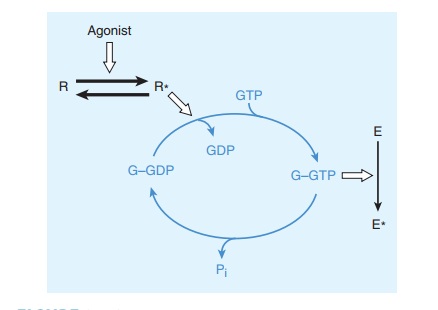
Gs
and other G proteins use a molecular mechanism that involves binding and
hydrolysis of GTP (Figure 2–10). This mechanism allows the transduced signal to
be amplified. For example, a neurotransmitter such as norepinephrine may
encoun-ter its membrane receptor for only a few milliseconds. When the
encounter generates a GTP-bound Gs molecule, however, the duration
of activation of adenylyl cyclase depends on the longevity of GTP binding to Gs
rather than on the receptor’s affinity for norepinephrine. Indeed, like other G
proteins, GTP-bound Gs may remain active for tens of seconds,
enormously amplifying the original signal. This mechanism also helps explain
how signaling by G proteins produces the phenomenon of spare receptors. The
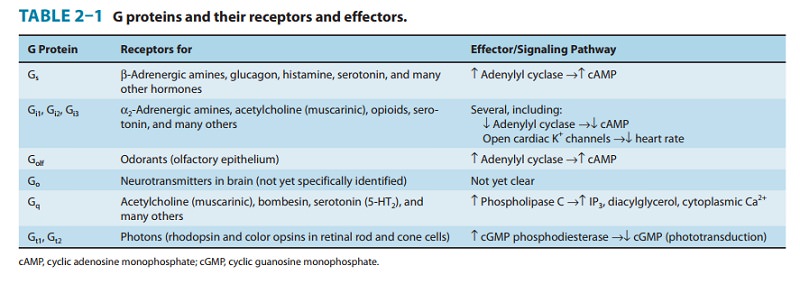
family of G proteins contains several functionally diverse sub-families (Table
2–1), each of which mediates effects of a particular set of receptors to a
distinctive group of effectors. Note that an endogenous ligand (eg,
norepinephrine, acetylcholine, serotonin, many others not listed in Table 2–1)
may bind and stimulate receptors that couple to different subsets of G
proteins. The appar-ent promiscuity of such a ligand allows it to elicit
different G protein-dependent responses in different cells. For instance, the
body responds to danger by using catecholamines (norepinephrine and
epinephrine) both to increase heart rate and to induce con-striction of blood
vessels in the skin, by acting on Gs-coupled β adrenoceptors and Gq-coupled
α1 adrenoceptors, respectively. Ligand promiscuity also offers
opportunities in drug development (see Receptor Classes & Drug Development
in the following text).
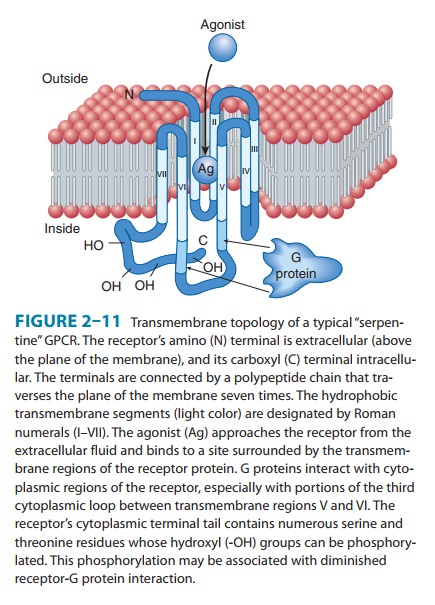
Receptors
coupled to G proteins are often called “G protein-coupled receptors” (GPCRs), “seven-transmembrane” (7-TM),
or “serpentine” receptors. GPCRs make up the largest receptor fam-ily and are
so-named because the receptor polypeptide chain “snakes” across the plasma
membrane seven times (Figure 2–11). Receptors for adrenergic amines, serotonin,
acetylcholine (musca-rinic but not nicotinic), many peptide hormones, odorants,
and even visual receptors (in retinal rod and cone cells) all belong to the
GPCR family. All were derived from a common evolutionary precursor. A few GPCRs
(eg, GABAB and metabotropic glutamate receptors) require stable
assembly into either homodimers
(com-plexes of two identical receptor polypeptides) or heterodimers (complexes of different isoforms) for functional
activity. However, in contrast to tyrosine kinase and cytokine receptors, most
GPCRs are thought to be able to function as monomers.
All
GPCRs transduce signals across the plasma membrane in essentially the same way.
Often the agonist ligand—eg, a cate-cholamine or acetylcholine—is bound in a
pocket enclosed by the transmembrane regions of the receptor (as in Figure
2–11). The resulting change in conformation of these regions is trans-mitted to
cytoplasmic loops of the receptor, which in turn acti-vate the appropriate G
protein by promoting replacement of GDP by GTP, as described above. Amino acids
in the third cyto-plasmic loop of the GPCR polypeptide are generally thought to
play a key role in mediating receptor interaction with G proteins (shown by
arrows in Figure 2–11). The structural basis for ligand binding to β adrenoceptors was
determined recently using X-ray crystallography.
Related Topics