Chapter: Basic & Clinical Pharmacology : Drug Receptors & Pharmacodynamics
Ligand-Regulated Transmembrane Enzymes Including Receptor Tyrosine Kinases
Ligand-Regulated Transmembrane
Enzymes Including Receptor Tyrosine Kinases
This
class of receptor molecules mediates the first steps in signaling by insulin,
epidermal growth factor (EGF), platelet-derived growth factor (PDGF), atrial
natriuretic peptide (ANP), trans-forming growth factor-β (TGF-β), and many other trophic hor-mones. These
receptors are polypeptides consisting of an extracellular hormone-binding
domain and a cytoplasmic enzyme domain, which may be a protein tyrosine kinase,
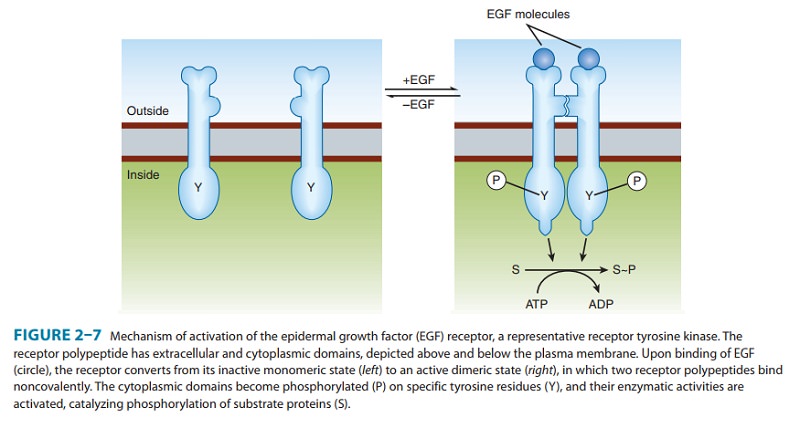
a serine kinase, or a guanylyl cyclase (Figure 2–7). In all these receptors,
the two domains are connected by a hydrophobic segment of the polypep-tide that
crosses the lipid bilayer of the plasma membrane.
The
receptor tyrosine kinase signaling pathway begins with binding of ligand,
typically a polypeptide hormone or growth fac-tor, to the receptor’s
extracellular domain. The resulting change in receptor conformation causes two
receptor molecules to bind to one another (dimerize),
which in turn brings together the tyrosine kinase domains, which become enzymatically
active, and phos-phorylate one another as well as additional downstream
signaling proteins. Activated receptors catalyze phosphorylation of tyrosine
residues on different target signaling proteins, thereby allowing a single type
of activated receptor to modulate a number of bio-chemical processes. (Some
receptor tyrosine kinases form oligo-meric complexes larger than dimers upon
activation by ligand, but the pharmacologic significance of such higher-order
complexes is presently unclear.)
Insulin,
for example, uses a single class of receptors to trigger increased uptake of
glucose and amino acids and to regulate metabolism of glycogen and
triglycerides in the cell. Similarly, each of the growth factors initiates in
its specific target cells a complex program of cellular events ranging from
altered mem-brane transport of ions and metabolites to changes in the
expres-sion of many genes.
Inhibitors
of receptor tyrosine kinases are finding increased use in neoplastic disorders
in which excessive growth factor signaling is often involved. Some of these
inhibitors are monoclonal anti-bodies (eg, trastuzumab, cetuximab), which bind
to the extracel-lular domain of a particular receptor and interfere with
binding of growth factor. Other inhibitors are membrane-permeant “small
molecule” chemicals (eg, gefitinib, erlotinib), which inhibit the receptor’s
kinase activity in the cytoplasm.
The
intensity and duration of action of EGF, PDGF, and other agents that act via
receptor tyrosine kinases are limited by a processcalled receptor down-regulation. Ligand binding often
induces accelerated endocytosis of receptors from the cell surface, followed by
the degradation of those receptors (and their bound ligands). When this process
occurs at a rate faster than de novo synthesis of receptors, the total number
of cell-surface receptors is reduced (down-regulated), and the cell’s
responsiveness to ligand is corre-spondingly diminished. A well-understood
example is the EGF receptor tyrosine kinase, which undergoes rapid endocytosis
fol-lowed by proteolysis in lysosomes after EGF binding; genetic mutations that
interfere with this process cause excessive growth factor-induced cell
proliferation and are associated with an increased susceptibility to certain
types of cancer. Endocytosis of other receptor tyrosine kinases, most notably
receptors for nerve growth factor, serves a very different function.
Internalized nerve growth factor receptors are not rapidly degraded and are
translo-cated in endocytic vesicles from the distal axon, where receptors are
activated by nerve growth factor released from the innervated tissue, to the
cell body. In the cell body, the growth factor signal is transduced to
transcription factors regulating the expression of genes controlling cell
survival. This process effectively transports a critical survival signal from
its site of release to its site of signaling effect, and does so over a
remarkably long distance—up to 1 meter in certain sensory neurons.
A
number of regulators of growth and differentiation, including TGF-β, act on another class
of transmembrane receptor enzymes that phosphorylate serine and threonine
residues. ANP, an impor-tant regulator of blood volume and vascular tone, acts
on a trans-membrane receptor whose intracellular domain, a guanylyl cyclase,
generates cGMP . Receptors in both groups, like the receptor tyrosine kinases,
are active in their dimeric forms.
Related Topics