Chapter: Biology of Disease: Diet and Disease
Vitamins - Investigating Nutritional Disorders
VITAMINS
The majority of vitamin disorders encountered in clinical
practice are deficiencies. The investigative procedures are varied and depend
upon the vitamin in question. Chemical tests can help to confirm the diagnosis
of overt vitamin deficiencies and may enable diagnosis to be made at a
relatively early stage. The types of tests used include direct measurements of
the concentration of the vitamin or one of its metabolites in plasma, serum,
erythrocytes, urine or tissue biopsies. The concentration of vitamin in plasma
does not necessarily reflect body vitamin status and a measurement of the
concentration in blood cells may be a better indicator. Enzyme-based tests are
available for some vitamins. Metabolites that accumulate in the blood or urine
following the blockage of a metabolic pathway normally catalyzed by an enzyme
that requires a vitamin as a cofactor or coenzyme may also be investigated.
Vitamin B1 (thiamin) deficiency can be assessed by
direct measurement of its concentration in plasma or indirectly by determining
the increase in erythrocyte transketolase activity in the presence of added
TPP. The increase in activity is called the activation coefficient. A
coefficient less than 15% is considered normal; an increase of 15–25% indicates
a marginal deficiency, while an increase greater than 25% with clinical signs
is indicative of severe thiamin deficiency. Thiamin deficiency can also be
assessed by the clinical response to administered thiamin, that is, an
improvement in the condition after administering thiamin supplements. The
nutritional status of vitamin B2 (riboflavin) is investigated in a
similar manner by determining the activation of glutathione reductase activity
of erythrocytes in the presence of added FAD.
Assessing the nutritional status of niacin is more difficult.
The usual method is to determine the concentrations of metabolites of niacin,
for example 1-methylnicotinamide and 1-methyl-3-carboxamido-6-pyridone, in
urine samples. Both are reasonably good measures of niacin status, as is the
ratio of the concentrations of NAD+ to NADP+ in
erythrocytes. A ratio of less than 1.0 may identify subjects at risk of
developing a niacin deficiency. The nutritional status of vitamin B5
(pantothenic acid) can also be assessed by determining its concentration in
plasma or urine samples. In general, plasma pantothenic acid concentrations
decrease in patients on a pantothenic acid deficient diet. However, the
concentrations of pantothenic acid in blood respond less readily to intake than
does the concentration in urine.
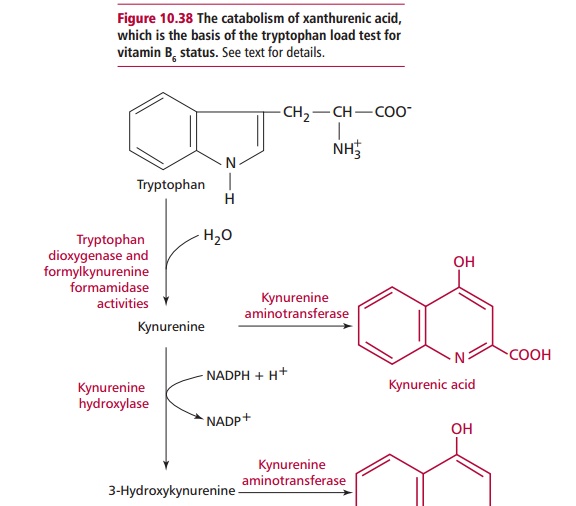
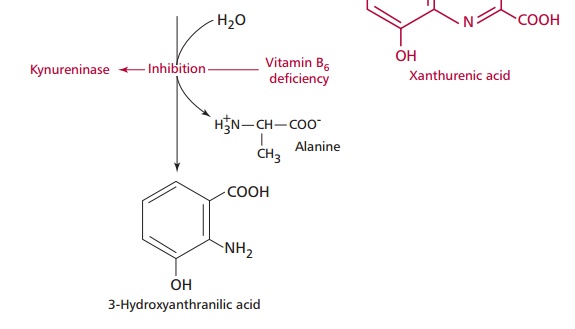
The status of vitamin B6 can be investigated in a
manner similar to those for thiamin and riboflavin by determining the
activation coefficients of erythrocyte alanine and aspartate transaminase
activities (ALT and AST respectively) in the presence of the cofactor pyridoxal
phosphate. Alternatively, vitamin B6 status may be assessed by the
tryptophan loading test. Tryptophan is normally catabolized by the pathway
shown in Figure 10.38. However, the
activity of kynureninase decreases markedly in B6 deficient
patients. If such a patient is given an oral dose of 50 mg per kilogram body
weight of tryptophan, then there is an increase in the amounts of kynurenic and
xanthurenic acids formed and these appear in the urine. Generally less than 30
mg of xanthurenic acid is excreted daily; higher amounts are indicative of
vitamin B6 deficiency. However, some other disorders of tryptophan
catabolism can also lead to an increase in xanthurenic acid production so
abnormal results must be treated with caution.
A possible deficiency of vitamin H (biotin) can be investigated by measuring its concentration in whole blood, serum or urine. Determining plasma biotin is not a reliable indicator of status. Changes in urinary excretion of biotin or of its metabolites are better indicators of biotin status.
Folic acid status may be investigated by directly measuring its
concentration in serum or erythrocytes although these are associated with a
number of problems. Serum folic acid tends to reflect dietary intake over the
previous few weeks. Patients with both acquired and inherited folic acid
deficiency may remain moderately deficient for months or years, taking in just
enough folic acid to prevent low erythrocyte folic acid concentrations and
frank anemia. Erythrocyte values are not sensitive to short-term variations;
depletion occurs only in the later stages of deficiency and is usually
accompanied by megaloblastic anemia. Both erythrocyte and serum folic acid
studies must be performed. Severe folic acid deficiency is accompanied by a
macrocytic anemia although the size of erythrocytes may be entirely normal in
lesser degrees of depletion. Serum vitamin B12 concentrations should
also be measured when evaluating folic acid deficiency since if either vitamin
is deficient it can lead to a failure in absorption by megaloblastic intestinal
cells resulting in a secondary deficiency of the other. Formiminoglutamic acid
(FIGLU) is a substrate for the folic acid dependent enzyme, formimino-glutamate
formiminotransferase required for histidine catabolism. When the vitamin is
deficient, FIGLU accumulates and is excreted into urine providing a sensitive
test of deficiency. However, FIGLU also increases in vitamin B12
deficiency and liver disease, so a high FIGLU excretion is not specific for the
diagnosis.
A deficiency of vitamin B12 (cobalamin) is
investigated by measuring its serum concentration and by hematological
examination of blood and bone marrow slides. Serum B12 can be
measured in isolation or as part of a Schilling test to exclude intrinsic
factor deficiency (pernicious anemia). A Schilling test will assess whether
vitamin B12 is being absorbed correctly by the body. The amount of
vitamin B12 excreted in urine over a 24 h period is determined after
giving the patient a known amount of radioactively labeled vitamin B12.
If the GIT is able to absorb vitamin B12 normally, then up to 25% of
the vitamin will be present in the urine. If there is failure in absorption,
then little or no vitamin B12 is detected in the urine. In the
latter case, the test is repeated following an oral dose of intrinsic factor to
determine whether the vitamin deficiency is due to lack of intrinsic factor or
a GIT problem.
The concentration of vitamin C in the plasma is a poor indicator
of deficiency. Measurements of cellular stores, especially in leukocytes are
more useful. However, vitamin C concentrations in leukocytes should always be
accompanied by a differential leukocyte count, given that different types of
leukocytes vary in their capacity to accumulate the vitamin. A change in the
proportion of polymorphonuclear leukocytes, which become saturated with vitamin
C at lower concentrations than other leukocytes, would result in a change in
total concentration of vitamin C per 106 cells, even if the
nutritional status of the vitamin is unchanged.
The concentration of vitamin A in the plasma can be measured but
this may be misleading as it only declines when tissue stores become severely
depleted. Deficiency can also occur in severe protein deficiency which
decreases the amount of its carrier protein. In such cases, the concentration
of plasma vitamin A would increase once the protein deficiency was corrected.
Clinical investigations of possible vitamin D deficiency involve determining
serum calcium and phosphate concentrations and measuring serum alkaline
phosphatase activity, since the enzyme is lost from cells during bone
catabolism. The metabolite, 25-hydroxycholecalciferol in samples of plasma can
be measured directly and is a good indicator of vitamin D status in the
presence of normal renal function. Vitamin E status can also be assessed by
direct measurement of its concentration in the plasma or serum, while vitamin K
deficiency is investigated by assessing the prothrombin time of the patient .
Related Topics