Chapter: Biology of Disease: Diet and Disease
Vitamins - Diet and Nutrition
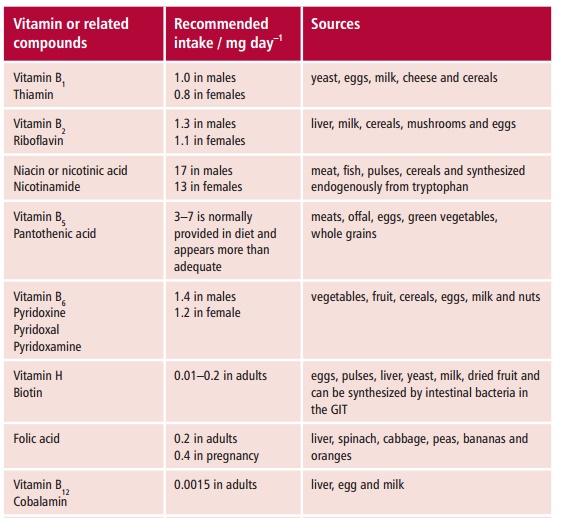
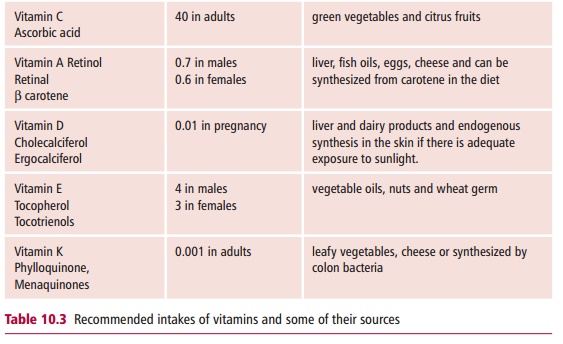
VITAMINS
Vitamins are organic substances that the body cannot
synthesize or can make only from chemically closely related compounds. They are
needed in the diet but only in relatively small amounts (Table 10.3).
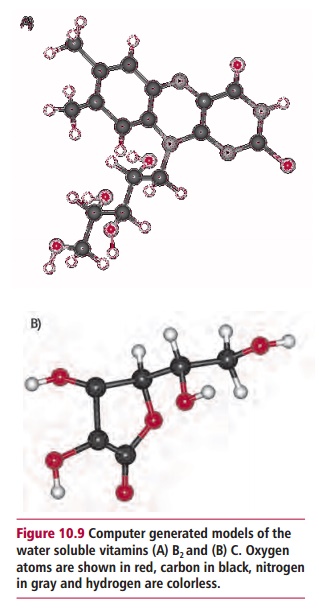
Vitamins may be classified as water soluble, such as
the B group and vitamin C (Figure 10.9)
and fat soluble, such as the vitamins A, D, E and K. Some of the B vitamins
and, in general, each fat soluble vitamin comprise a group of closely related
compounds called vitamers. In such
cases, the name of the vitamin is used as a collective descriptor. Vitamins
have specific biochemical roles and are essential for normal metabolism, growth
and good health.
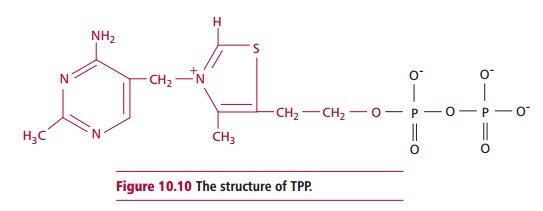
Vitamin B1 or thiamin is
an essential component of the coenzyme or prosthetic group, thiamin
pyrophosphate (TPP, Figure 10.10).
This is necessary for the actions of some enzymes, for example transketolase
activity of the pentose phosphate pathway for oxidizing glucose and oxidative
decarboxylations catalyzed by pyruvate and 2-oxoglutarate dehydrogenases in
carbohydrate metabolism. Thiamin is therefore necessary for the metabolic
formation of ATP, the major energy carrier in metabolism, and NADPH. Thiamin
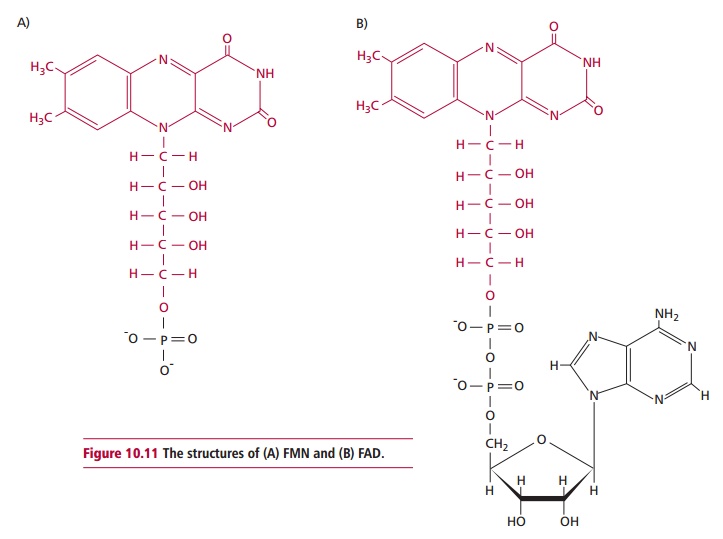
pyrophosphate is also known to function in nerve conductance. Vitamin B2 or riboflavin
is also required to form coenzymes or prosthetic groups, in this case flavin
mononucleotide (FMN, Figure 10.11 (A))
and flavin dinucleotide (FAD, Figure10.11
(B)), which function as electron carriers in flavoproteins. The
flavoproteinsare extremely numerous and include renal L-amino acid oxidase, NADH reductase, 2-hydroxyacid
oxidase (FMN) and D- and L-amino acid oxidases, succinate dehydrogenase and
glutathione reductases (FAD). Hence riboflavin is essential to many oxidation
and reduction reactions in, for example, the TCA cycle, electron transport and
the oxidation of fatty acids in mitochondria.
Nicotinic acid and nicotinamide are vitamers of
niacin, which strictly is not a vitamin since limited amounts of it can be
synthesized from tryptophan. Niacin is required to form coenzymes NAD+ and NADP+ (Figure 10.12) whichare electron and
hydrogen carriers. The former is crucial in electron transport associated with
oxidative phosphorylation and ATP formation, the reduced form of NADPH is
essential to the biosynthesis of, for example sugars, lipids, amino acids and
nucleotides.
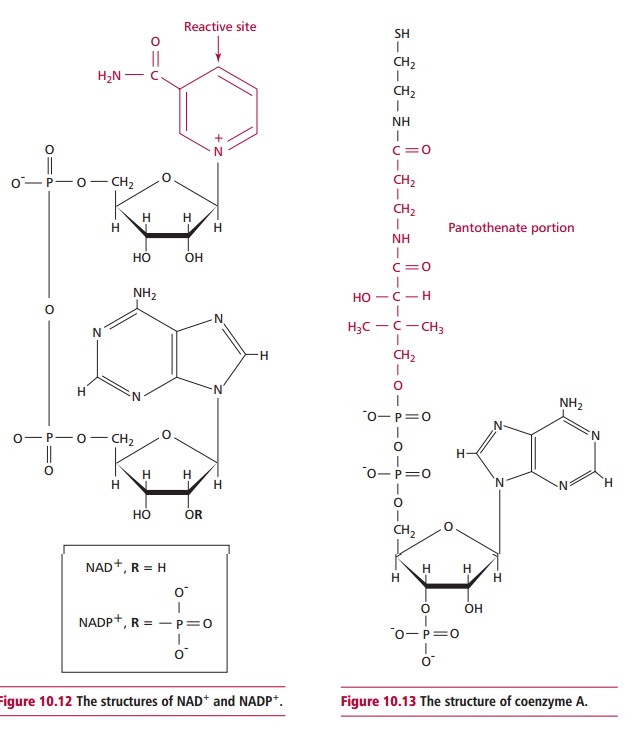
Vitamin B5 or pantothenic
acid is an essential part of coenzyme A (Figure
10.13), which is the major carrier of metabolically active acyl (fatty acid
residues) in metabolism. Thus it is essential to many of the reactions involved
in the oxidation of lipids and in the synthesis of lipids including steroid
hormones, some neurotransmitters and hemoglobin.
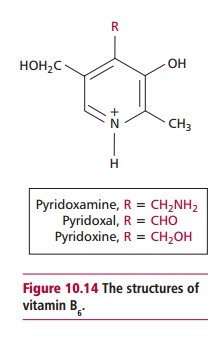
The vitamers pyridoxine, pyridoxal and pyridoxamine
all have vitamin B6 activity (Figure 10.14), forming pyridoxal 5-phosphate, which is a cofactor
for a number of enzymes. These include glutamate decarboxylase that catalyzes
the formation of F aminobutyric acid, a neurotransmitter of the central
nervous system, and enzymes that catalyze transamination and decarboxylation reactions
of amino acids. Vitamin B6 is therefore essential for the
synthesis of nonessential amino acids and in the catabolism of amino acids.
Pyridoxal 5-phosphate is also a cofactor for glycogen phosphorylase of liver
and muscle tissues and helps regulate the actions of steroid hormones by
participating in the dissociation of hormone–receptor complexes from DNA.
Vitamin B12 or cobalamin
is an unusual molecule in that it contains an organometallic bond between
cobalt and carbon (Figure 13.18 (A)).
A close relationship exists between the functions of vitamin B12 and folic acid
and, to some extent at least, they depend on each other for activation. Organic
one carbon groups, for example methyl (CH3–), methylene (–CH2-), methenyl
(–CH=), formyl (–CHO), formate (–COO–) and formino (–CHNH), are
generally toxic. In metabolism, they are bound to carriers derived from vitamin
B12 and folic acid, which allows them to be converted to different
oxidation states and used in a variety of different reactions in a nontoxic
manner. These reactions are necessary for the catabolism of some amino acids,
for the formation of a number of proteins and the synthesis of purine and
pyrimidine bases and therefore nucleotides and nucleic acids. Unlike vitamin B12
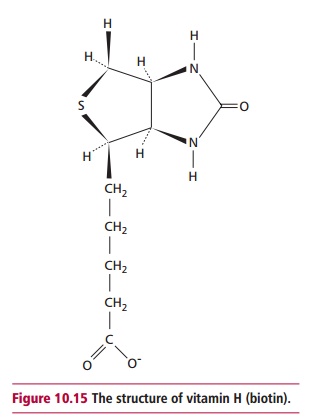
and folic acid that carry organic one carbon compounds, vitamin H or biotin (Figure 10.15) is required to form the
prosthetic group that carries CO2 in a number of enzymes. These
include acetyl CoA carboxylase and pyruvate decarboxylase which are key enzymes
of fatty acid synthesis and gluconeogenesis, the production of glucose from
noncarbohydrate precursors.
Vitamin C or ascorbic acid (Figure
10.16) is required to reduce metal ions in a number of enzymes following
catalysis. Prolyl and lysyl hydroxylases contain Fe2+(II) which is
oxidized to Fe3+(III) during hydroxylation reactions involved in
cross-linking collagen molecules, which adds strength to connective tissues.
Ascorbate reduces their iron back to the ferrous state, regenerating an active
enzyme. Similarly, the Cu2+(II) in enzymes involved in synthesis of
catecholamine hormones is returned to the Cu+(I) state following the
oxidation of the copper during catalysis. The antioxidant properties of
ascorbic acid, in association with vitamin E, help protect lipids in the cell
membranes and blood lipoproteins from oxidative damage. It also enhances the
absorption of iron and regulates the absorption of copper from the GIT.
Several vitamers, retinol, retinaldehyde or retinal and retinoic
acid, show vitamin A activities. Retinol (Figure
10.17 (A)) can be metabolically converted to retinaldehyde, which, in turn
can be oxidized to retinoic acid. In addition, the provitamin A carotenoids,
for example A carotene (Figure
10.17 (B)), can be converted to active forms in the liver. Retinoic acid
helps regulate the proliferation and development of cells in a tissue specific
manner that resembles the actions of steroid hormones . It binds to nuclear
receptors, which then interact with DNA and activate specific genes. Vitamin A
is associated with the development of epithelial cells, such as the skin and
the mucosal membranes that cover internal and external surfaces of the body and
have numerous functions, for example, as structural barriers that
prevent microorganisms from entering the body . Retinaldehyde is
necessary for vision and functions as the prosthetic group (visual pigments) of
opsin proteins in light-sensitive retinal cells. Vitamin A is a weak
antioxidant that can protect against free radical damage. There is some
evidence that A carotene reduces the incidence of cardiovascular
disease and some forms of cancer.
The usual dietary form of vitamin D is cholecalciferol (Figure 10.18), although this is not
strictly a vitamin since it can be formed by ultraviolet irradiation of the
skin from 7-dehydrocholesterol. Foods are sometimes fortified with the
synthetic ergocalciferol, which has the same biological activity as
cholecalciferol. Enzyme-catalyzed hydroxylations yield the active metabolites 1@, 25 dihydroxycholecalciferol and calcitriol
respectively. Vitamin D mainly functions in the homeostasis of calcium as
described.
Vitamin E is generally used to describe the tocopherols and
tocotrienols that comprise a number of vitamers of differing biological
potencies, of which the most active is @ tocopherol (Figure 10.19
(A) and (B)). Like vitamins A and
D, vitamin E also has a role in regulating gene expression, although a receptor
has yet to be found, and also in signal transduction. A major function
generally ascribed to vitamin E is to protect cellular membranes against free
radicals and prevent the oxidation of
plasma lipoproteins, especially low density lipoproteins . The corresponding
reduction of the vitamin produces a relatively unreactive and therefore less
damaging tocopheroxyl radical. This is also relatively long lived and so
persists sufficiently long to be reoxidized back to the active form by vitamin
C or glutathione peroxidases. However, the utility of this mechanism has been
challenged and it has been suggested that the antioxidant role is more
restricted and arises from an inhibition of NADPH oxidase and so reduces the
production of radicals, such as superoxide.
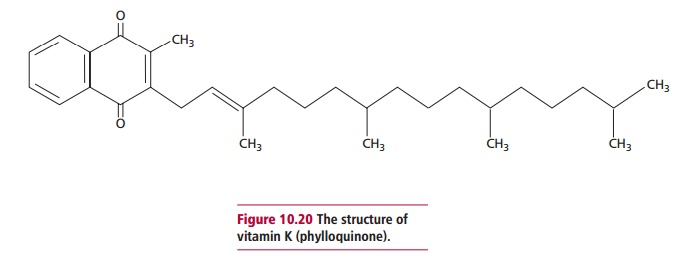
A number of compounds are possible vitamers of vitamin K,
including phylloquinone and the menaquinones. The first is the normal dietary
source, while the menaquinones are a group of compounds with similar structures
synthesized by GIT bacteria. Phylloquinone (Figure
10.20) is metabolically necessary for the conversion of glutamate residues
in some proteins to F carboxyglutamates. This is
necessary for the synthesis of some blood clotting factors, which are
described, and some proteins of the bone matrix. The ability of menaquinones to
function as vitamin K is unclear; they may partially satisfy the human
requirements for vitamin K but their contribution is probably much less than
previously thought.
Related Topics