Chapter: Plant Biochemistry: A leaf cell consists of several metabolic compartments
Translocators catalyze the specific transport of metabolic substrates and products
Translocators catalyze the specific transport of metabolic substrates and products
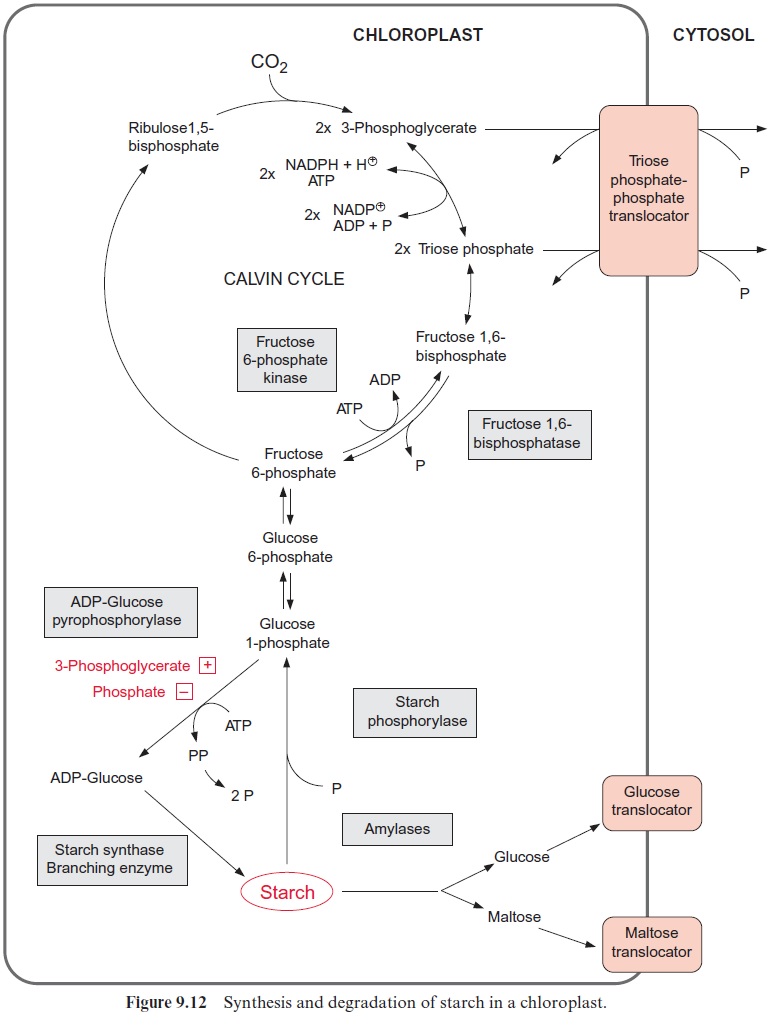
Specialized membrane proteins catalyze a specific transport across membranes. In the past these proteins were called carriers, as it was assumed that after binding the substrate at one side of the membrane, they would diffuse through the membrane to release the substrate on the other side. We now know that this simple picture does not apply. Instead, transport can be visualized as a process by which a molecule moves through a specific pore. The proteins catalyzing such a transport are termedtranslocators or transporters. The triose phosphate-phosphate translocator of chloroplasts will be used as an example to describe the structure and function of such a translocator. This translocator enables the export of photoassimilates from the chloroplasts by catalyzing a counter-exchange of phosphate with triose phosphate (dihydroxyacetone phosphate or glyceraldehyde-3-phos-phate) (Fig. 9.12). Quantitatively it is the most abundant transport protein in plants.
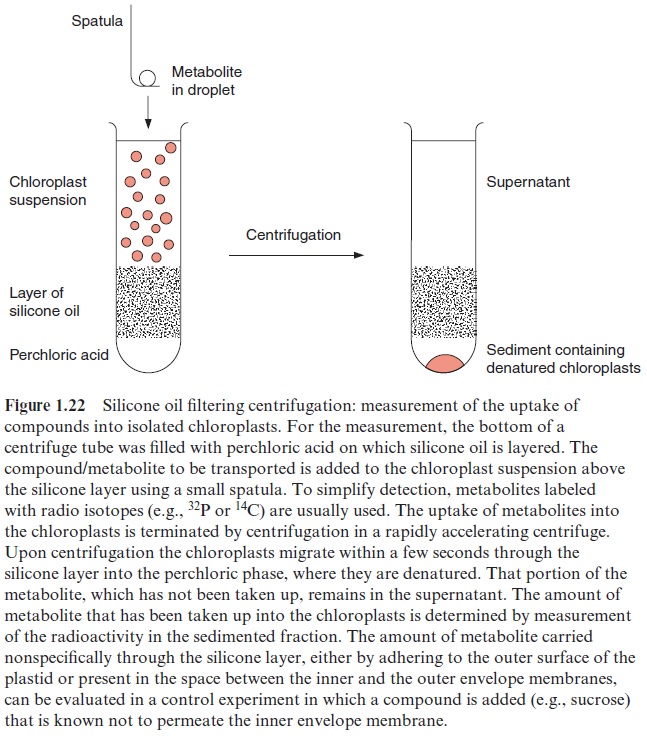
Silicone layer filtering centrifugation is a very useful tool (Fig. 1.22) for measuring the uptake of substrates into chloroplasts or other cell organelles. To start measurement of transport, the corresponding substrate is added to a suspension of isolated chloroplasts and is terminated by sepa-rating the chloroplasts from the surrounding medium by centrifugation through a silicone layer. The amount of substrate taken up into the sepa-rated chloroplasts is then quantitatively analyzed.
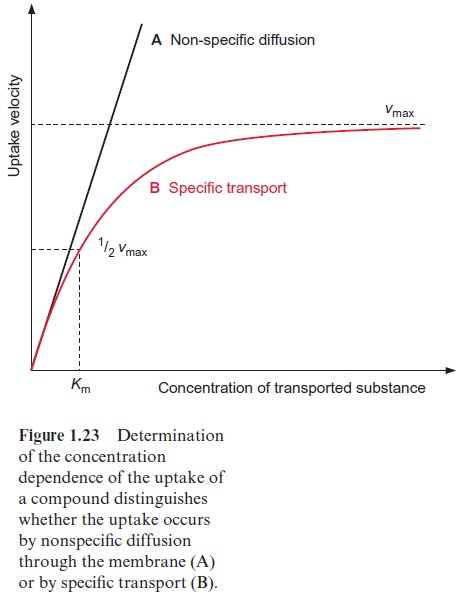
A hyperbolic curve is observed (Fig. 1.23) when this method is used to measure the uptake of phosphate into chloroplasts at various external con-centrations of phosphate. At very low phosphate concentrations the rate of uptake rises proportionally to the external concentration, whereas at higher phosphate concentrations the curve levels off until a maximal velocity is reached (Vmax). These are the same characteristics as seen in enzyme catalysis.
During enzyme catalysis the substrate (S) is first bound to the enzyme (E).
The product (P) formed on the enzyme surface is then released:
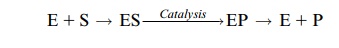
E + S → ES →( catalysis) → EP → E + P
The transport by a specific translocator can be depicted in a similar way:

S + T → ST →( Transport) → TS → T + S
The substrate is bound to a specific binding site of the translocator pro-tein (T), transported through the membrane, and then released from the translocator. The maximal velocity Vmax corresponds to a state in which all the binding sites of the translocators are saturated with substrate. As is the case for enzymes, the Km for a translocator corresponds to the sub-strate concentration at which transport occurs at half maximum velocity. Also in analogy to enzyme catalysis, the translocators usually show highspecificity for the transported substrates. For instance, the chloroplast tri-ose phosphate-phosphate translocator (trioseP-P translocator) of C3 plants transports orthophosphate, dihydroxyacetone phosphate, glyc-eraldehyde-3-phosphate, and 3-phosphoglycerate, but not 2-phosphoglyc-erate. The various substrates compete for the binding site. Therefore, one substrate such as phosphate will be a competitive inhibitor for the transport of another substrate such as 3-phosphoglycerate. The trioseP-P transloca-tor of chloroplasts is an antiporter, so that for each molecule transported inward (e.g., phosphate), another molecule (e.g., dihydroxyacetone phos-phate) must be transported out of the chloroplasts. Another example for an antiporter is the mitochondrial ATP-ADP translocator which transports ADP and ATP, but not AMP, phosphate or other nucleotides.
Metabolite transport is achieved by a conformational change of the translocator
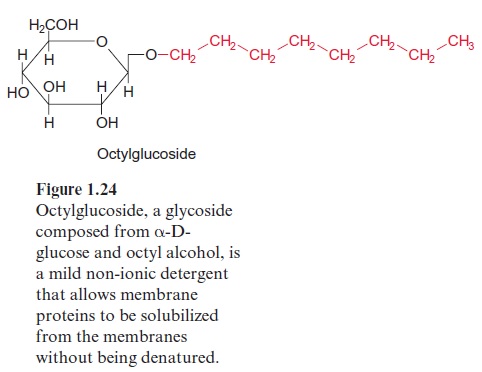
Translocators are, as integral membrane proteins, part of a membrane. Due to their high hydrophobicity they are not soluble in water, which made studies of their protein structure difficult. In order to isolate these proteins from the membranes by solubilization, mild nonionic detergents, such as octylglucoside (Fig. 1.24), are employed. The hydrophobic hydrocarbon chain of the detergent associates with the hydrophobic protein. Because of the glucose residue of octylglucoside, the formed micelle is water soluble. A removal of the detergent would turn the membrane protein into a sticky mass, which could not be solubilized again.
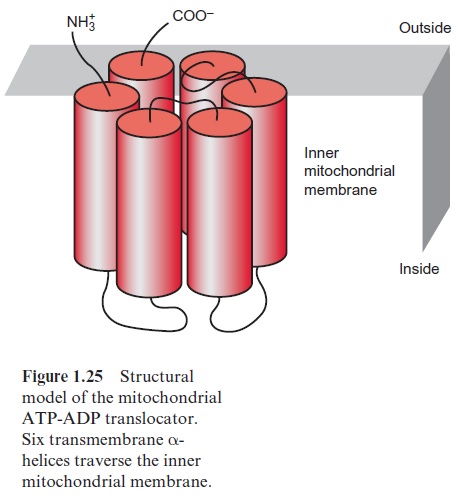
Translocators traverse the lipid bilayer of a membrane via α-helices, of which the outside directed amino acid side chains are hydrophobic. The transmembrane helices contain predominantly hydrophobic amino acids such as alanine, valine, leucine, isoleucine or phenylalanine. Figure 1.25 shows a structural model of the monomer of the mitochondrial ATP-ADP translocator. Six transmembrane helices span the inner mitochondrial mem-brane, and loops directed to the inside cause the specificity of the trans-port. The mitochondrial ATP-ADP translocator occurs in the membrane as a dimer of identical monomers (homodimer). The X-ray structure analysis of the mitochondrial ATP/ADP translocator revealed for the first time the three-dimensional structure of a eukaryotic metabolite translo-cator. It appeared that the six transmembrane helices of the monomer form a barrel-like structure functioning as a translocation pore. Consequently, the homodimer consists of two adjacent identical pores. Similarly, the chloroplast trioseP-P translocator occurs in the membrane as a homodimer, but it is not yet certain how many transmembrane helices the monomer consists of.
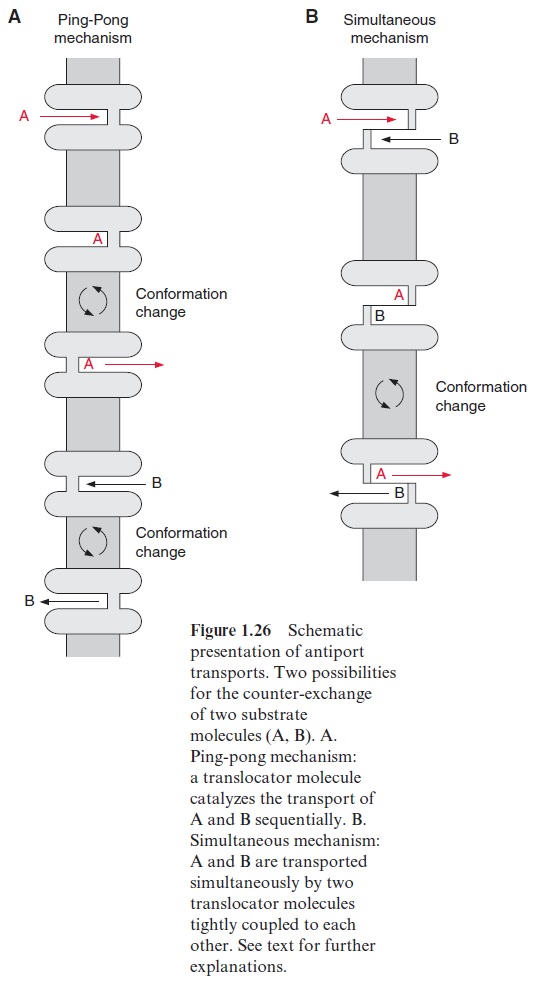
As discussed earlier, the translocation pores are gated, each contain-ing only one substrate binding site, accessible either from the outside or from the inside, whereas the accessibility is governed by the conformation of the translocator protein (Fig. 1.26). The transport process resembles a gate. The binding of a substrate (A) to a binding site directed to the outside induces a conformational change of the translocator by which the substrate binding site is shifted to the inside, enabling the release of the substrate. The now empty binding site at the inside can bind another substrate (B), inducing a conformational change for B to be transported to the outside. An obligatory counter-exchange can be explained in terms of the opening of the gate by shifting the binding sites via conformational change being possible only when the substrate binding site is occupied. This principle of an antiport has been termed ping-pong mechanism (Fig. 1.26A), and prob-ably describes the action of the chloroplast trioseP-P translocator. In many cases, e.g., the mitochondrial ATP-ADP translocator, the counter-exchange follows a simultaneous mechanism (Fig. 1.26B), where the substrate bind-ing sites of the adjacent pores of the dimer are oppositely directed; one pore is accessible from the inside and the other from the outside, and a simultaneous conformational change occurs only when both binding sites are occupied.
A plant contains a large number of such metabolite translocators. The analysis of the Arabidopsis genome suggests that altogether about 150 plas-tidic and 60 mitochondrial translocator genes exist.
Aquaporins make cell membranes permeable for water
The water permeability of a pure lipid bilayer is relatively low. Peter Agre from the Johns Hopkins University in Baltimore isolated from kidney and blood cells proteins that form membrane channels for water, which he termed aquaporins. In 2003 he was awarded the Nobel Prize in Chemistry for this important discovery. It turned out that these aquaporins also occur in plants (e.g., in plasma membranes and membranes of the vacuole). Notably both types of membranes play a major role in the hydrodynamic response of a plant cell. A plant contains many aquaporin isoforms. Thus in the model plant Arabidopsis thaliana about 35 different genes of the aquaporin family have been found, which are specifically expressed in the various plant organs.
X-ray structure analysis by electron cryomicroscopy showed that the subunits of the aquaporins each have six transmembrane helices (sec-tion 3.3). In the membranes the subunits of the aquaporins are present as tetramers, of which each monomer forms a channel, transporting 109 to 1011 water molecules per second. The water channelconsists of a very narrow primarily hydrophobic pore with binding sites for only seven H2O molecules. These binding sites act as a selection filter for a specific water transport. It can be deduced from the structure that, for energetic reasons, these water channels are relatively impermeable for protons. It was also observed that an aquaporin from the plasma membrane of tobacco also transports CO2. This finding suggests that aquaporins may play a role as CO2 transporters in plants.
By regulating the opening of the aquaporins, the water conductivity of the plasma membrane can be adjusted to the environmental conditions. The aquaporins of plants possess a peptide loop which is able to close the water channel like a lid via conformational change. In this way the water channels can be closed upon drought stress or flooding. During drought stress the closure of the water channel is caused by dephosphorylation of two highly conserved serine residues. In the case of flooding or waterlog-ging the accompanying oxygen deficiency (anoxy) results in a decrease of the pH in the cytosol, causing the protonation of a histidine residue, which in turn induces a conformational change of the channel protein closing the lid of the water channel.
The now commonly used term aquaporin is rather unfortunate, since aquaporins have an entirely different structure from the porins. Whereas the aquaporins, the translocators and ion channels are formed of transmembrane helices, the porins consist of β-sheets .
Related Topics