Chapter: Physics : Effects of electric current : Higher Secondary(12 Std)
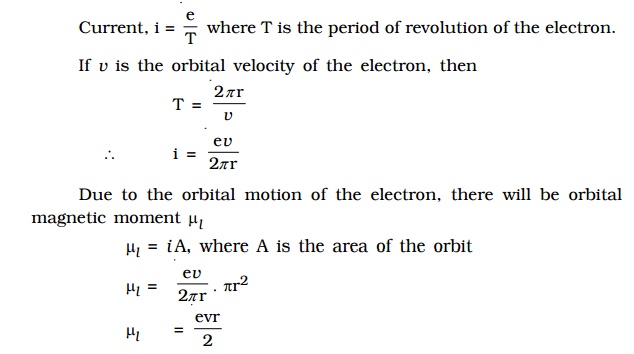
The magnetic dipole moment of a revolving electron
The
magnetic dipole moment of a revolving electron
According to Neil Bohr’s atom model,
the negatively charged electron is revolving around a positively charged
nucleus in a circular orbit of radius r.
The revolving electron in a closed path constitutes an electric current. The
motion of the electron in anticlockwise direction produces conventional current
in clockwise direction.
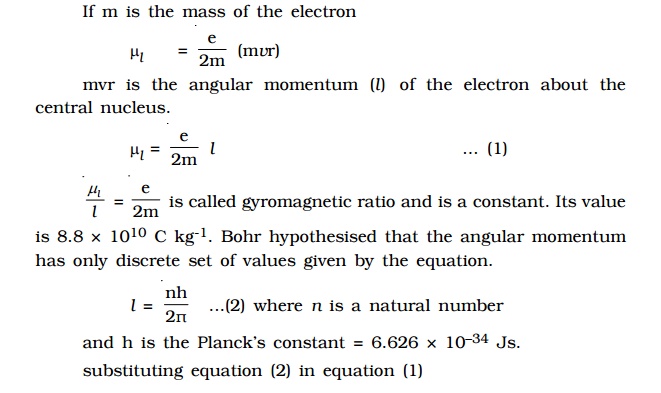
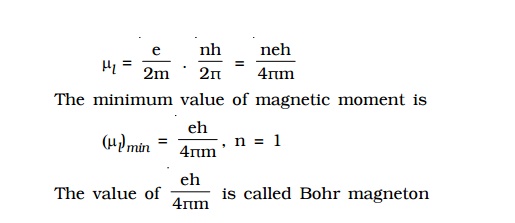
By
substituting the values of e, h and m, the value of Bohr magneton is found
to be 9.27 × 10–24 Am2
In addition to the magnetic moment
due to its orbital motion, the electron possesses magnetic moment due to its
spin. Hence the resultant magnetic moment of an electron is the vector sum of
its orbital magnetic moment and its spin magnetic moment.
Related Topics