Chapter: Biochemistry: Lipids and Proteins Are Associated in Biological Membranes
The Chemical Natures of the Lipid Types
The Chemical Natures of the Lipid
Types
What are fatty acids?
A fatty
acid has a carboxyl group at the polar end and a hydrocarbon chain at the
nonpolar tail. Fatty acids are amphipathic
compounds because the carboxyl group is hydrophilic and the hydrocarbon tail is
hydrophobic. The carboxyl group can ionize under the proper conditions.
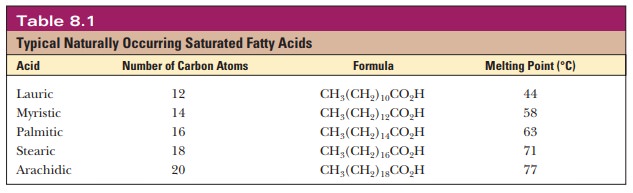
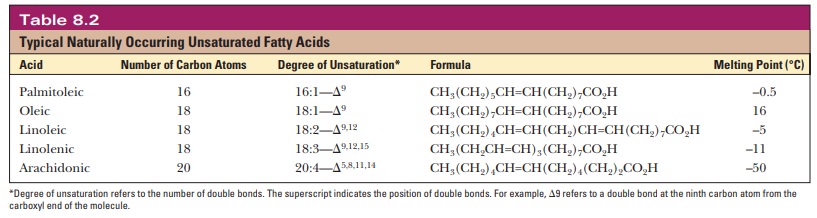
A fatty acid that occurs in a living system normally contains an even number of carbon atoms, and the hydrocarbon chain is usually unbranched (Figure 8.1). If there are carbon–carbon double bonds in the chain, the fatty acid is unsaturated; if there are only single bonds, the fatty acid is saturated. Tables 8.1and 8.2 list a few examples of the two classes. In unsaturated fatty acids, the stereochemistry at the double bond is usually cis rather than trans. The differ-ence between cis and trans fatty acids is very important to their overall shape. A cis double bond puts a kink in the long-chain hydrocarbon tail, whereas the shape of a trans fatty acid is like that of a saturated fatty acid in its fully extended conformation. Note that the double bonds are isolated from one another by several singly bonded carbons; fatty acids do not normally have conjugated double-bond systems.
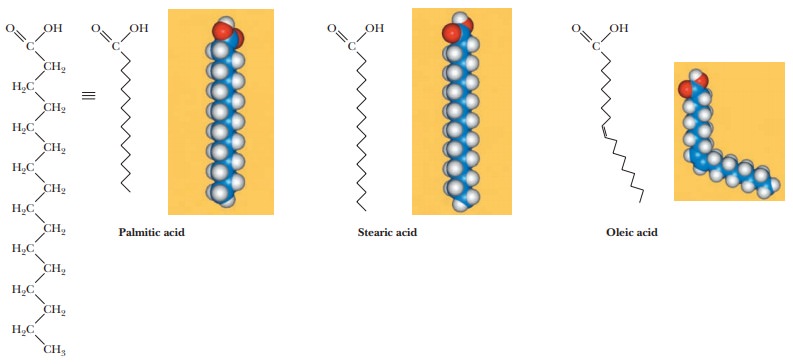
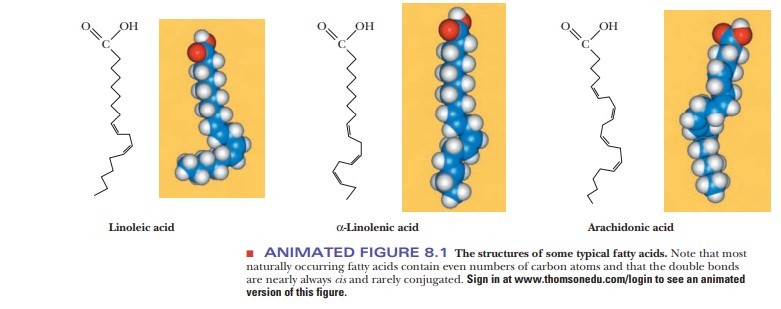
The notation used for fatty acids indicates the number of carbon atoms and the number of double bonds. In this system, 18:0 denotes an 18-carbon saturated fatty acid with no double bonds, and 18:1 denotes an 18-carbon fatty acid with one double bond. Note that in the unsaturated fatty acids in Table 8.2 (except arachidonic acid), there is a double bond at the ninth car-bon atom from the carboxyl end. The position of the double bond results from the way unsaturated fatty acids are synthesized in organisms. Unsaturated fatty acids have lower melting points than saturated ones. Plant oils are liquid at room temperature because they have higher proportions of unsaturated fatty acids than do animal fats, which tend to be solids. Conversion of oils to fats is a commercially important process. It involves hydrogenation, the process of adding hydrogen across the double bond of unsaturated fatty acids to produce the saturated counterpart. Oleomargarine, in particular, uses partially hydrogenated vegetable oils, which tend to include trans fatty acids.
Fatty
acids are rarely found free in nature, but they form parts of many com-monly
occurring lipids.
What are triacylglycerols?
Glycerol is a simple compound that contains three hydroxyl groups
(Figure8.2). When all three of the alcohol groups form ester linkages with
fatty acids, the resulting compound is a triacylglycerol;
an older name for this type of compound is triglyceride.
Note that the three ester groups are the polar part of the molecule, whereas
the tails of the fatty acids are nonpolar. It is usual for three different
fatty acids to be esterified to the alcohol groups of the same glycerol
molecule. Triacylglycerols do not occur as components of membranes (as do other
types of lipids), but they accumulate in adipose tissue (primarily fat cells)
and provide a means of storing fatty acids, particularly in animals. They serve
as concentrated stores of metabolic energy. Complete oxidation of fats yields
about 9 kcal g–1, in contrast with 4 kcal g–1 for
carbohydrates and proteins.
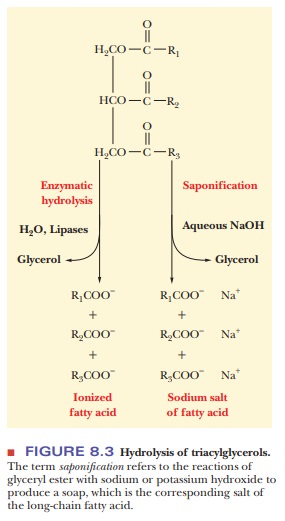
When an organism uses fatty acids, the ester linkages of triacylglycerols are hydrolyzed by enzymes called lipases. The same hydrolysis reaction can take place outside organisms, with acids or bases as catalysts. When a base such as sodium hydroxide or potassium hydroxide is used, the products of the reac-tion, which is called saponification (Figure 8.3), are glycerol and the sodium or potassium salts of the fatty acids. These salts are soaps. When soaps are used with hard water, the calcium and magnesium ions in the water react with the fatty acids to form a precipitate-the characteristic scum left on the insides of sinks and bathtubs. The other product of saponification, glycerol, is used in creams and lotions as well as in the manufacture of nitroglycerin.
What are phosphoacylglycerols?
It is
possible for one of the alcohol groups of glycerol to be esterified by a
phosphoric acid molecule rather than by a carboxylic acid. In such lipid
molecules, two fatty acids are also esterified to the glycerol molecule. The
resulting compound is called a phosphatidic
acid (Figure 8.4a). Fatty acids are usually monoprotic acids with only one
carboxyl group able to form an ester bond, but phosphoric acid is triprotic and
thus can form more than one ester linkage. One molecule of phosphoric acid can
form ester bonds both to glycerol and to some other alcohol, creating a phosphatidyl ester (Figure 8.4b).
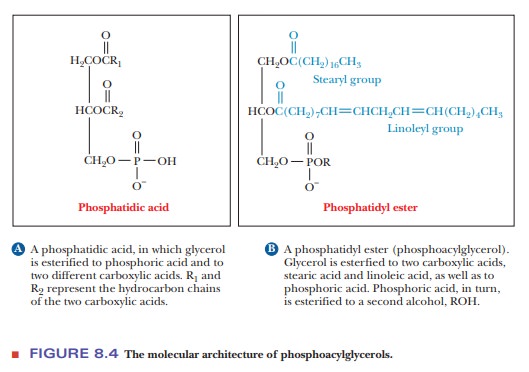
Phosphatidyl esters are classed as phosphoacylglycerols. The natures of the fatty acids vary widely, as they do in triacylglycerols. As a result, the names of the types of lipids (such as triacylglycerols and phosphoacylglycerols) that contain fatty acids must be considered generic names.
The
classification of a phosphatidyl ester depends on the nature of the second
alcohol esterified to the phosphoric acid. Some of the most impor-tant lipids
in this class are phosphatidyl
ethanolamine (cephalin), phosphatidylserine,
phosphatidyl choline (lecithin),
phosphatidyl inositol, phosphatidyl glycerol, and diphosphatidyl glycerol (cardiolipin) (Figure 8.5). In each of
these types of compounds, the nature of the fatty acids in the molecule can
vary widely.
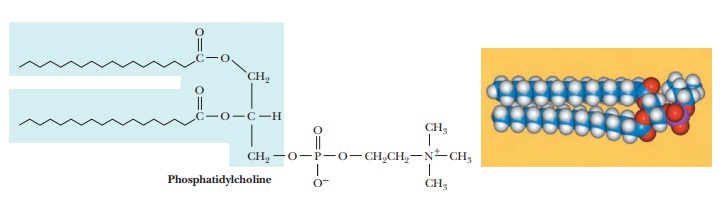
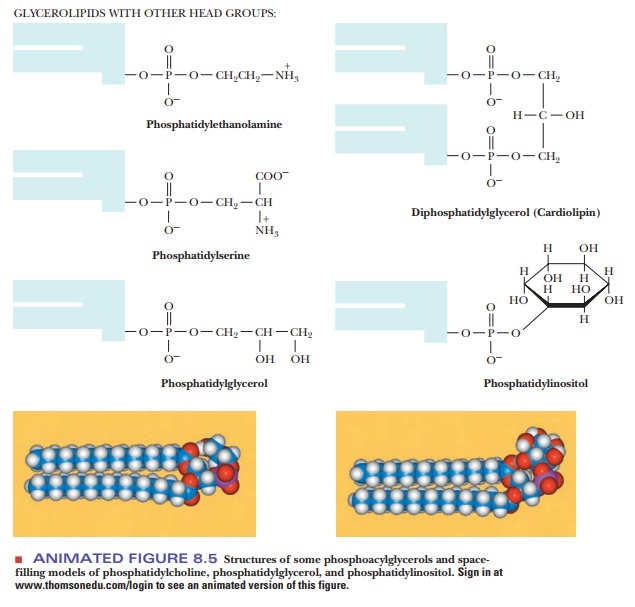
All these
compounds have long, nonpolar, hydrophobic tails and polar, highly hydrophilic
head groups and thus are markedly amphipathic. (We have already seen this
characteristic in fatty acids.) In a phosphoacylglycerol, the polar head group
is charged, because the phosphate group is ionized at neutral pH. A positively
charged amino group is also frequently contributed by an amino alcohol
esterified to the phosphoric acid. Phosphoacylglycerols are important
components of biological membranes.
What are waxes and sphingolipids?
Waxes are complex mixtures of
esters of long-chain carboxylic acids and long-chain alcohols. They frequently
serve as protective coatings for both plants and animals. In plants, they coat
stems, leaves, and fruit; in animals, they are found on fur, feathers, and
skin. Myricyl cerotate (Figure 8.6), the principal component of carnauba wax,
is produced by the Brazilian wax palm. Carnauba wax is extensively used in floor
wax and automobile wax. The principal component of spermaceti, a wax produced
by whales, is cetyl palmitate (Figure 8.6). The use of spermaceti as a
component of cosmetics made it one of the most highly prized products of
19th-century whaling efforts.
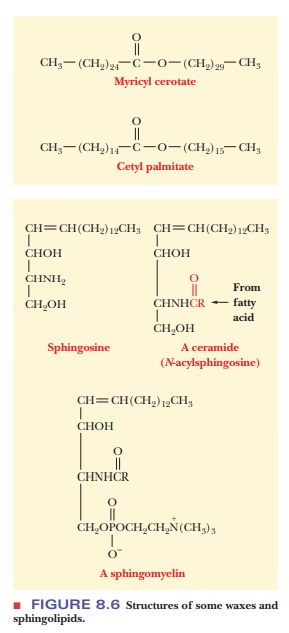
Sphingolipids do not contain glycerol, but
they do contain the long-chainamino alcohol sphingosine, from which this class
of compounds takes its name (Figure 8.6). Sphingolipids are found in both
plants and animals; they are par-ticularly abundant in the nervous system. The
simplest compounds of this class are the ceramides, which consist of one fatty
acid linked to the amino group of sphingosine by an amide bond (Figure 8.6). In
sphingomyelins, the primary alcohol
group of sphingosine is esterified to phosphoric acid, which, in turn, is
esterified to another amino alcohol, choline (Figure 8.6). Note the structural
similarities between sphingomyelin and other phospholipids. Two long
hydro-carbon chains are attached to a backbone that contains alcohol groups. One
of the alcohol groups of the backbone is esterified to phosphoric acid. A
second alcohol-choline, in this case-is also esterified to the phosphoric acid.
We have already seen that choline occurs in phosphoacylglycerols.
Sphingomyelins are amphipathic; they occur in cell membranes in the nervous
system (see the following Biochemical Connections box).
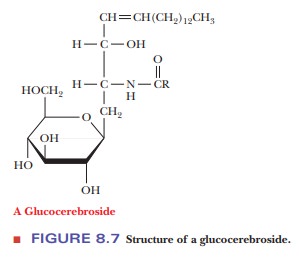
What are glycolipids?
If a
carbohydrate is bound to an alcohol group of a lipid by a glycosidic linkage,
the resulting compound is a glycolipid.
Quite frequently, ceramides (see
Figure 8.6) are the parent compounds for glycolipids, and the glycosidic bond
is formed between the primary alcohol group of the ceramide and a sugar
residue. The resulting compound is called a cerebroside. In most cases, the sugar is glucose or galactose; for
example, a glucocerebroside is a cerebroside that contains glucose (Figure
8.7). As the name indicates, cerebrosides are found in nerve and brain cells,
primarily in cell membranes. The carbohydrate portion of these compounds can be
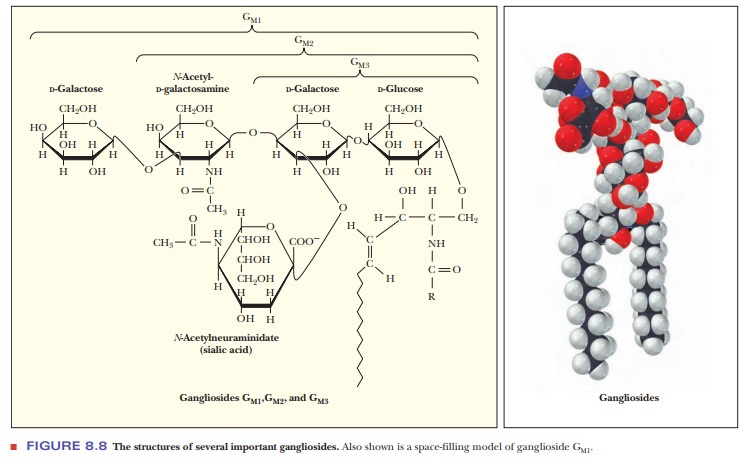
very complex. Gangliosides are examples of glycolipids with a complex
carbohydrate moiety that contains more than three sugars. One of them is always
a sialic acid (Figure 8.8). These compounds are also referred to as acidic
glycosphingolipids because of their net negative charge at neutral pH.
Glycolipids are often found as markers on cell membranes and play a large role
in tissue and organ specificity. Gangliosides are also present in large
quantities in nerve tissues.
What are steroids?
Many
compounds of widely differing functions are classified as steroids because they have the same general structure: a fused-ring
system consisting of three
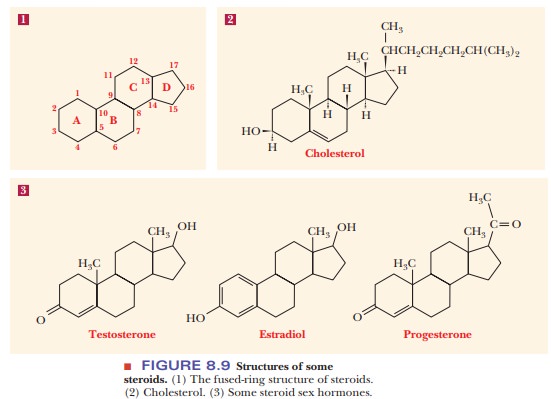
There are many important steroids, including sex hormones. The steroid that is of most interest in our discussion of membranes is cholesterol (Figure 8.9). The only hydrophilic group in the cholesterol structure is the single hydroxyl group. As a result, the molecule is highly hydrophobic. Cholesterol is widespread in biological membranes, especially in animals, but it does not occur in prokaryotic cell membranes.
The presence of cholesterol in membranes can modify the role of
membrane-bound proteins. Cholesterol has a number of important biological
functions, including its role as a precursor of other steroids and of vitamin D3. We
will see a five-carbon structural motif (the isoprene unit) that is common to
steroids and to fat-soluble vitamins, which is an indication of their
biosynthetic relationship. However, cholesterol is best known for its harmful
effects on health when it is present in excess in the blood. It plays a role in
the development of atherosclerosis, a
condition in which lipid deposits block the blood vessels and lead to heart
disease.
Summary
Lipids are frequently
open-chain compounds with a polar head group and a long nonpolar tail.
Glycerol, fatty acids, and
phosphoric acid are frequently obtained as deg-radation products of lipids.
Another
class of lipids consists of fused-ring compounds called steroids.
Related Topics