Chapter: Biochemistry: Lipids and Proteins Are Associated in Biological Membranes
Lipid-Soluble Vitamins and Their Functions
Lipid-Soluble Vitamins and Their
Functions
What is the role of lipid-soluble vitamins in the body?
Some
vitamins, having a variety of functions, are of interest because they are
soluble in lipids. These lipid-soluble vitamins are hydrophobic, which accounts
for their solubility (Table 8.3).
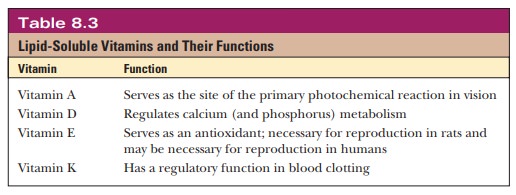
Vitamin A
The
extensively unsaturated hydrocarbon β-carotene
is the precursor of vitaminA, which
is also known as retinol. As the
name suggests,b-carotene is abundantin carrots, but it also occurs in other
vegetables, particularly the yellow ones. When an organism requires vitamin A,
β-carotene is converted to the vitamin (Figure 8.28).
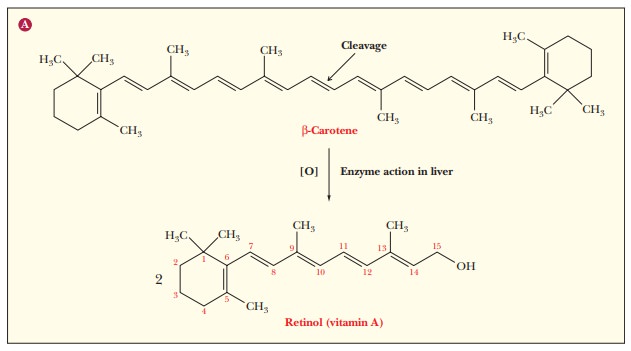
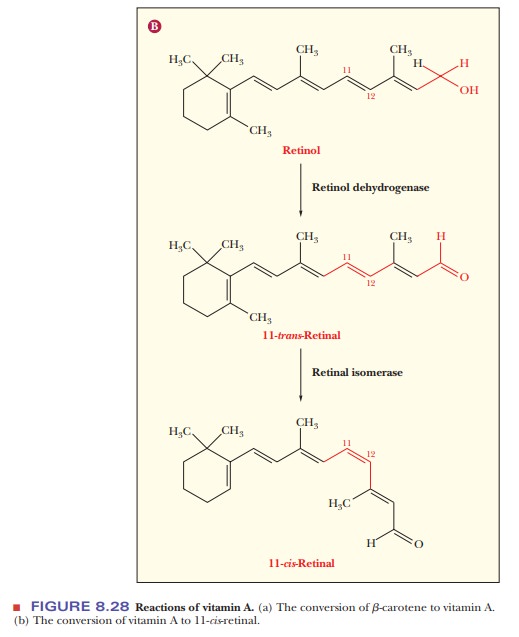
A
derivative of vitamin A plays a crucial role in vision when it is bound to a
protein called opsin. The cone cells
in the retina of the eye contain several types of opsin and are responsible for
vision in bright light and for color vision. The rod cells in the retina
contain only one type of opsin; they are responsible for vision in dim light.
The chemistry of vision has been more extensively studied in rod cells than in
cone cells, and we shall discuss events that take place in rod cells.
Vitamin
A has an alcohol group that is enzymatically oxidized to an aldehyde group, forming
retinal (Figure 8.28b). Two isomeric
forms of retinal, involving cis–trans isomerization
around one of the double bonds, are important in thebehavior of this compound
in vivo. The aldehyde group of retinal forms an imine (also called a Schiff
base) with the side-chain amino group of a lysine residue in rod-cell opsin
(Figure 8.29).
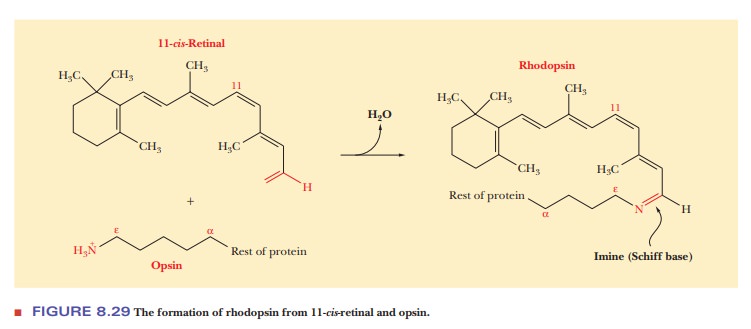
The
product of the reaction between retinal and opsin is rhodopsin. The outer segment of rod cells contains flat membrane
enclosed discs, the mem-brane consisting of about 60% rhodopsin and 40% lipid.
(For more details about rhodopsin, see the following Biochemical Connections
box.)
Vitamin D
The
several forms of vitamin D play a
major role in the regulation of calcium and phosphorus metabolism. One of the
most important of these compounds, vitamin D3
(cholecalciferol), is formed from cholesterol by the action of ultraviolet
radiation from the Sun. Vitamin D3 is further processed in the
body to form hydroxylated derivatives, which are the metabolically active form
of this vitamin (Figure 8.30). The presence of vitamin D3 leads
to increased synthesis of a Ca2+-binding protein, which
increases the absorption of dietary calcium in the intestines. This process
results in calcium uptake by the bones.
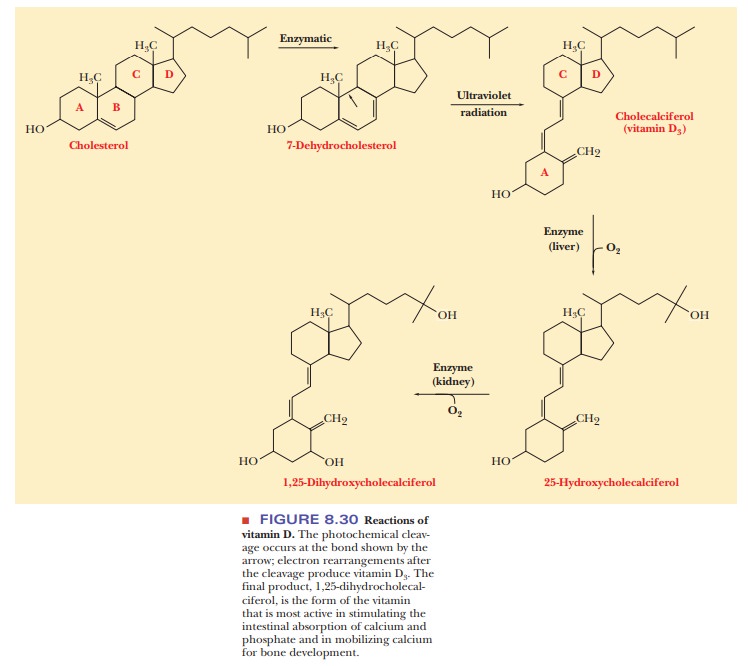
A
deficiency of vitamin D can lead to rickets,
a condition in which the bones of growing children become soft, resulting in
skeletal deformities. Children, especially infants, have higher requirements
for vitamin D than do adults. Milk with vitamin D supplements is available to
most children. Adults who are exposed to normal amounts of sunlight do not
usually require vitamin D supplements.
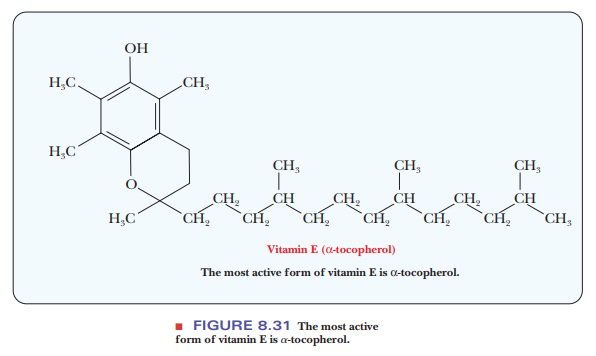
Vitamin E
The most active form of vitamin E is α-tocopherol (Figure 8.31). In rats, vitamin E is required for reproduction
and for prevention of the disease musculardystrophy.
It is not known whether this requirement exists in humans. A
well-established chemical property of vitamin E is that it is an antioxidant-that is, a good reducing
agent-so it reacts with oxidizing agents before they can attack other
biomolecules. The antioxidant action of vitamin E has been shown to protect
important compounds, including vitamin A, from degradation in the laboratory;
it probably also serves this function in organisms.
Recent
research has shown that the interaction of vitamin E with membranes enhances
its effectiveness as an antioxidant. Another function of antioxidants such as
vitamin E is to react with, and thus to remove, the very reactive and highly
dangerous substances known as free
radicals. A free radical has at least one unpaired electron, which accounts
for its high degree of reactivity. Free radicals may play a part in the
development of cancer and in the aging process.
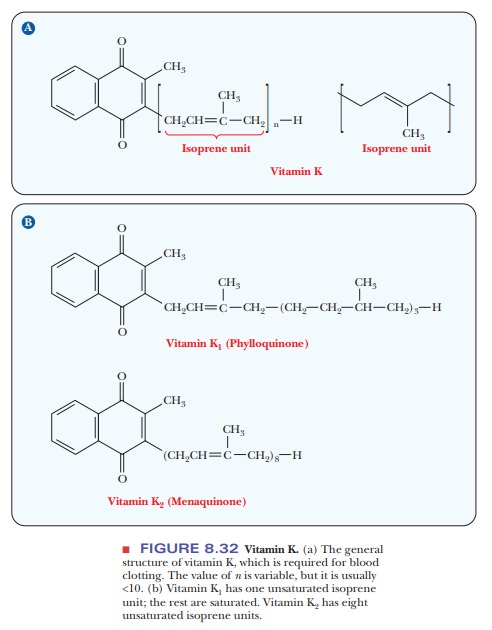
Vitamin K
The name
of vitamin K comes from the Danish Koagulation because this vitamin is an
important factor in the blood-clotting process. The bicyclic ring system
contains two carbonyl groups, the only polar groups on the molecule (Figure
8.32). A long unsaturated hydrocarbon side chain consists of repeating isoprene units, the number of which
determines the exact form of vitamin K. Several forms of this vitamin can be
found in a single organism, but the reason for this variation is not well
understood. Vitamin K is not the first vitamin we have encountered that
contains isoprene units, but it is the first one in which the number of
isoprene units and their degree of saturation make a difference. (Can you pick
out the isoprene-derived portions of the structures of vitamins A and E?) It is
also known that the steroids are biosynthetically derived from isoprene units,
but the structural relationship is not immediately obvious.
The
presence of vitamin K is required in the complex process of blood clot-ting,
which involves many steps and many proteins and has stimulated numer-ous
unanswered questions. It is known definitely that vitamin K is required to
modify prothrombin and other proteins involved in the clotting process.
Specifically, with prothrombin, the addition of another carboxyl group alters
the side chains of several glutamate residues of prothrombin. This
modifica-tion of glutamate produces γ-carboxyglutamate residues (Figure 8.33).
The two carboxyl groups in proximity form a bidentate
(“two teeth”) ligand, which can
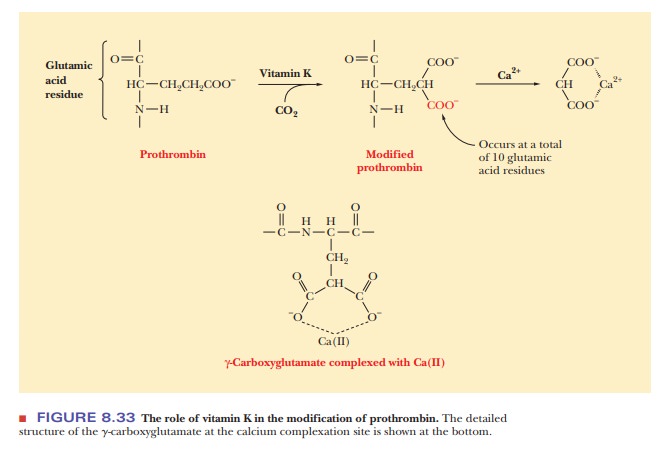
If prothrombin is not modified in this way, it does not bind Ca2+. Even
though there is a lot more to be learned about blood clotting and the role of
vitamin K in the process, this point, at least, is well established, because Ca2+ is
required for blood clotting. (Two well-known anticoagulants, dicumarol and
warfarin (a rat poison), are vitamin K antagonists.)
Summary
The structures of the
lipid-soluble vitamins-A, D, E, and K-are ulti-mately derived from five-carbon
isoprene units, which also play a role in lipid biosynthesis.
Vitamin
A plays a crucial role in vision. Vitamin D is necessary for bone integrity
because of its role in calcium and phosphorus metabolism. Vitamin E is an
important antioxidant, and vitamin K plays a role in blood clotting.
Related Topics