Chapter: Biochemistry: Lipids and Proteins Are Associated in Biological Membranes
Biological Membranes
Biological Membranes
Every
cell has a cell membrane (also called a plasma membrane); eukaryotic cells also
have membrane-enclosed organelles, such as nuclei and mitochondria. The
molecular basis of the membrane’s structure lies in its lipid and protein
components. Now it is time to see how the interaction between the lipid bilayer
and membrane proteins determines membrane function. Membranes not only separate
cells from the external environment but also play important roles in transport
of specific substances into and out of cells. In addition, a number of
important enzymes are found in membranes and depend on this environment for
their function.
Phosphoglycerides are prime examples of
amphipathic molecules, and theyare the principal lipid components of membranes.
The existence of lipid bilay-ers depends
on hydrophobic interactions, as described. Thesebilayers are frequently used as
models for biological membranes because they have many features in common, such
as a hydrophobic interior and an ability to control the transport of small
molecules and ions, but they are simpler and easier to work with in the
laboratory than biological membranes.
The most
important difference between lipid bilayers and cell membranes is that the
latter contain proteins as well as lipids. The protein component of a membrane
can make up from 20% to 80% of its total weight. An understand-ing of membrane
structure requires knowledge of how the protein and lipid components contribute
to the properties of the membrane.
What is the structure of lipid bilayers?
Biological membranes contain, in addition to phosphoglycerides, glycolipids as part of the lipid component. Steroids are present in eukaryotes-cholesterol in animal membranes and similar compounds, called phytosterols, in plants. In the lipid bilayer part of the membrane (Figure 8.10), the polar head groups are in contact with water, and the nonpolar tails lie in the interior of the membrane. The whole bilayer arrangement is held together by noncovalent interactions, such as van der Waals and hydrophobic interactions. The surface of the bilayer is polar and contains charged groups. The nonpolar hydrocarbon interior of the bilayer consists of the saturated and unsaturated chains of fatty acids and the fused-ring system of cholesterol.

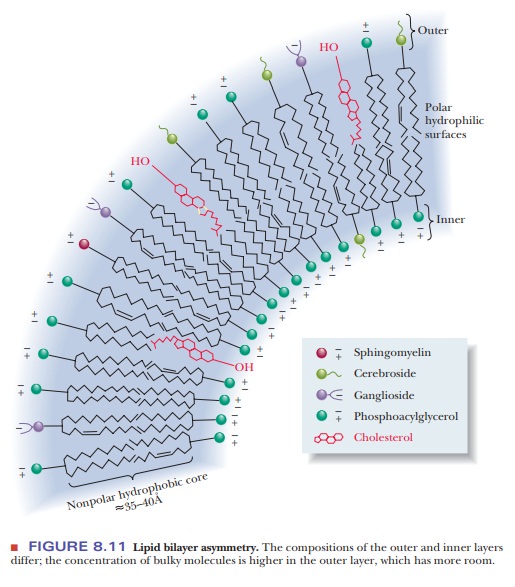
Both the
inner and outer layers of the bilayer contain mixtures of lipids, but their
compositions differ and can be used to distinguish the inner and outer layers
from each other (Figure 8.11). Bulkier molecules tend to occur in the outer
layer, and smaller molecules tend to occur in the inner layer.
How does the composition of the bilayer affect its properties?
The
arrangement of the hydrocarbon interior of the bilayer can be ordered and rigid
or disordered and fluid. The bilayer’s fluidity depends on its composition. In
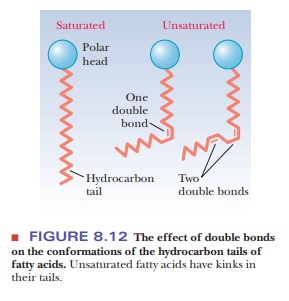
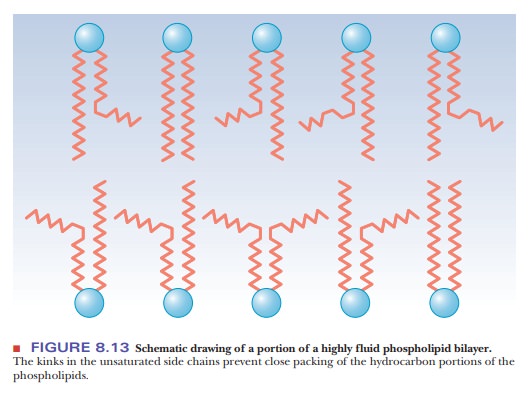
saturated fatty acids, a linear arrangement of the hydrocarbon chains leads to
close packing of the molecules in the bilayer, and thus to rigidity.
Unsaturated fatty acids have a kink in the hydrocarbon chain that does not
exist in saturated fatty acids (Figure 8.12). The kinks cause disorder in the
packing of the chains, which makes for a more open structure than would be
possible for straight saturated chains (Figure 8.13). In turn, the disordered
structure caused by the presence of unsaturated fatty acids with cis double bonds (and therefore kinks)
in their hydrocarbon chains causes greater fluidity in the bilayer. The lipid
components of a bilayer are always in motion, to a greater extent in more fluid
bilayers and to a lesser extent in more rigid ones.
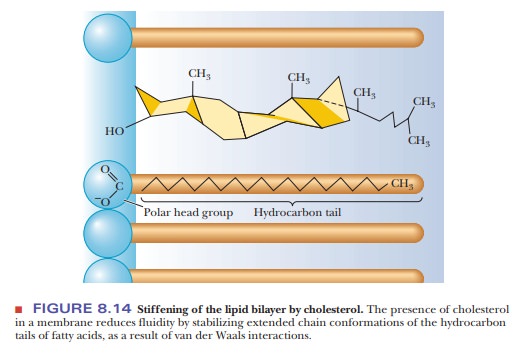
The
presence of cholesterol may also enhance order and rigidity. The fused-ring
structure of cholesterol is itself quite rigid, and the presence of cholesterol
stabilizes the extended straight-chain arrangement of saturated fatty acids by
van der Waals interactions (Figure 8.14). The lipid portion of a plant membrane
has a higher percentage of unsaturated fatty acids, especially polyunsaturated
(containing two or more double bonds) fatty acids, than does the lipid portion
of an animal membrane. Furthermore, the presence of cho-lesterol is
characteristic of animal, rather than plant, membranes. As a result, animal
membranes are less fluid (more rigid) than plant membranes, and the membranes
of prokaryotes, which contain no appreciable amounts of steroids, are the most
fluid of all. Research suggests that plant sterols can act as natural
cholesterol blockers, interfering with the uptake of dietary cholesterol.
With
heat, ordered bilayers become less ordered; bilayers that are compara-tively
disordered become even more disordered. This cooperative transition takes place
at a characteristic temperature, like the melting of a crystal, which
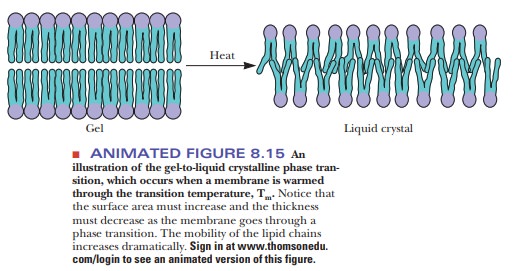
The transition temperature is higher
for more rigid and ordered membranes than it is for relatively fluid and
disordered membranes. The following Biochemical Connections box looks at some
connections between the fatty acid composition of bilayers and mem-branes and
how they behave at different temperatures.
Recall
that the distribution of lipids is not the same in the inner and outer portions
of the bilayer. Because the bilayer is curved, the molecules of the inner layer
are more tightly packed (refer to Figure 8.11). Bulkier molecules, such as
cerebrosides, tend to be located in the outer layer. There is very little
tendency for “flip-flop” migration of lipid molecules from one layer of the
bilayer to another, but it does occur occasionally. Lateral motion of lipid
molecules within one of the two layers frequently takes place, however,
especially in more fluid bilayers. Several methods exist for monitoring the
motions of molecules within a lipid bilayer. These methods depend on label-ing
some part of the lipid component with an easily detected “tag.” The tags are
usually fluorescent compounds, which can be detected with highly sensitive
equipment. Another kind of labeling method depends on the fact that some
nitrogen compounds have unpaired electrons. These compounds are used as labels
and can be detected by magnetic measurements.
Summary
Lipid bilayers are large assemblies of
molecules. The polar head groups of the lipid molecules are in contact with an
aqueous environment. The nonpolar tails of the lipids are out of contact with
the aqueous environ-ment. The bilayer is like a sandwich with polar head groups
as the bread and the nonpolar tails as the filling.
Bulkier molecules tend to be found in the outer layer, rather than
in the inner one.
The presence of saturated fatty acids and of cholesterol tends to
stiffen the bilayer.
The packing of molecules in the bilayer can undergo a reversible transi-tion from order to disorder.
Related Topics