Chapter: Biochemistry: Lipids and Proteins Are Associated in Biological Membranes
Butter versus Margarine-Which Is Healthier?
Butter versus Margarine-Which Is
Healthier?
We use
the terms animal “fats” and plant “oils” because of the solid and fluid nature
of these two groups of lipids. The major difference between fats and oils is
the percentage of unsaturated fatty acids in the triglycerides and the
phosphoglycerides of membranes. This difference is far more important than the
fact that the length of the fatty acid chain can affect the melting points.
Butter is an exception; it has a high proportion of short-chain fatty acids and
thus can “melt in your mouth.” Membranes must maintain a certain degree of
fluidity to be functional. Consequently, unsaturated fats are distributed in
varying proportions in different parts of the body. The membranes of internal
organs of warm-blooded mammals have a higher percentage of saturated fats than
do the membranes of skin tissues, which helps keep the membrane more solid at
the higher temperature of the internal organ. An extreme example of this is
found in the legs and the body of reindeer, where marked differences exist in
the percentages of saturated fatty acids.
When
bacteria are grown at different temperatures, the fatty acid composition of the
membranes changes to reflect more unsaturated fatty acids at lower temperatures
and more saturated fatty acids at higher temperatures. The same type of
difference can be seen in eukaryotic cells grown in tissue culture.
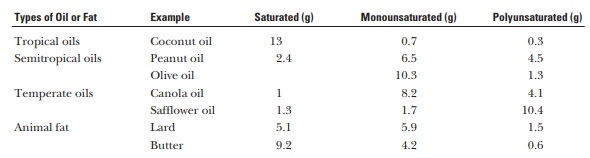
Even if
we look at plant oils alone, we find different propor-tions of saturated fats
in different oils. The following table gives the distribution for a tablespoon
(14 g) of different oils.
Because cardiovascular disease is correlated with diets high in saturated fats, a diet of more unsaturated fats may reduce the risk of heart attacks and strokes.
Canola oil is an attractive dietary choice because
it has a high ratio of unsaturated fatty acids to saturated fatty acids. Since
the 1960s, we have known that foods higher in polyunsaturated fats were
healthier. Unfortunately, even though olive oil is popular in cooking Italian
food and canola oil is trendy for other cooking, pouring oil on bread or toast
is not appealing. Thus companies began to market butter substitutes that were
based on unsaturated fatty acids but that would also have the physical
characteristics of butter, such as being solid at room temperature. They
accomplished this task by partially hydrogenating the double bonds in the
unsaturated fatty acids making up the oils. The irony here is that, to avoid
eating the saturated fatty acids in butter, butter substitutes were created
from polyunsaturated oils by removing some of the double bonds, thus making
them more saturated. In addition, many of the soft spreads that are marketed as
being healthy (safflower oil spread and canola oil spread) may indeed pose new
health risks. In the hydrogenation process, some double bonds are converted to
the trans form. Studies now show that trans fatty acids raise the ratioof LDL
(low-density lipoprotein) cholesterol compared to HDL (high-density
lipoprotein) cholesterol, a positive correlator of heart disease. Thus the
effects of trans fatty acids are
similar to those of saturated fatty acids. In the last few years, however, new
butter substitutes have been marketed that advertise “no trans fatty acids.”
Related Topics