Chapter: Genetics and Molecular Biology: Protein Structure
The Amino Acids - Protein Structure
The Amino Acids
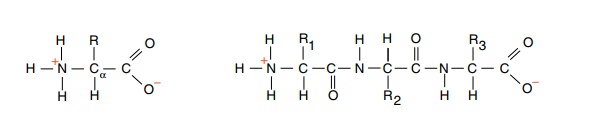
Proteins consist of α-L-amino acids linked by peptide bonds to form polypeptide chains (Fig.
6.1). At neutral pH, the carboxyl group of a free amino acid is negatively
charged and the amino group is positively charged. In a protein, however, these
charges are largely, but not completely absent from the interior amino acids
owing to the formation of the peptide bonds between the amino groups and
carboxyl groups. Of course, the N-terminal amino group of a protein is
positively charged and the C-terminal carboxyl group is negatively charged.
Figure 6.1 An α-L-amino acid with negative charge on the carboxyl and positive charge on the amino group and three amino acids linked by peptide bonds.
Twenty different types of α-L-amino acids are commonly found in proteins (Fig. 6.2). Except for
proline, which technically is an imino acid, these differ from one another only
in the structure of the side group attached to the alpha carbon. A few other
types of amino acids are occasionally found in proteins, with most resulting
from modification of one of the twenty after the protein has been synthesized.
Frequently these modified amino acids are directly involved with chemical
reac-tions catalyzed by the protein. Each of the basic twenty must possess
unique and invaluable properties since most proteins contain all twenty
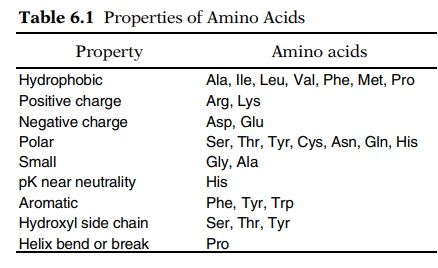
different amino acids (Table 6.1).
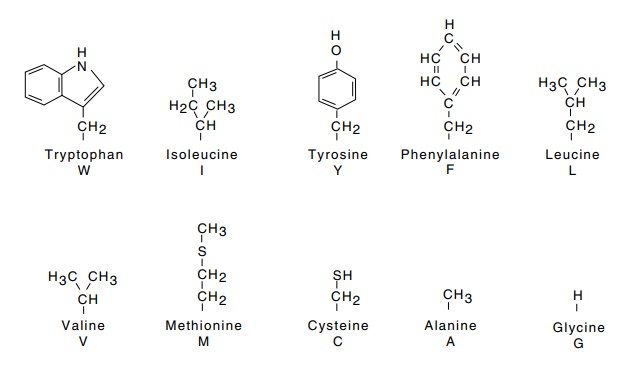
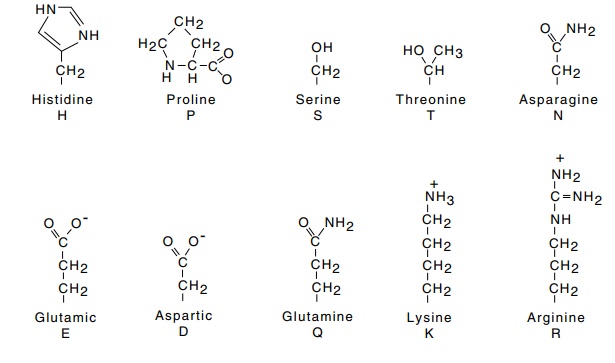
Figure
6.2 The side chains of amino acids
and their single letter abbreviations.The complete structure of proline is
shown. The most hydrophobic amino acids are at the top and the most hydrophilic
are at the bottom.
Even though we must understand the individual properties of each of the amino acids, it is convenient to classify the twenty into a smaller number of groups and to understand common
properties of the groups. One of the most important such groups is the
hydrophobics. The side groups of the aliphatic amino acids are hydrophobic and
prefer to exist in a nonaqueous, nonpolar environment like that found in the
contact
region between two subunits, in the portion of a
protein bound to a membrane, or in the interior of a globular protein. A
contiguous area of such amino acids on a portion of the surface can make a
protein bind
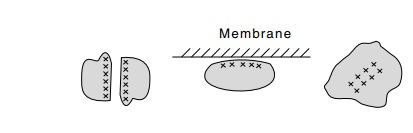
to a similar hydrophobic patch on the surface of
another protein, as in the oligomerization of protein subunits, or it can make
the protein prefer to bind to or even enter a membrane. Hydrophobic amino acids
on the interior of a protein prefer the company of one another to the exclusion
of water. This is one of the major forces that maintains the structure of a
folded protein.
The basic amino acid side groups of amino acids
like lysine and arginine possess a positive charge at neutral pH. If located on
the surface of the protein, such positive charges can assist the binding of a
nega-tively charged ligand, for example DNA. The acidic amino acid side groups
of glutamic acid and aspartic acid possess a negative charge at neutral pH.
Neutral amino acid side groups possess no net charge, and
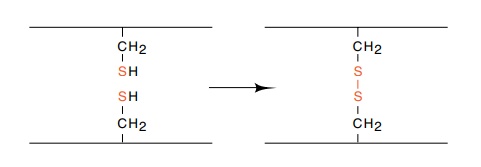
Figure 6.3
Two reduced cysteine residues and
their oxidized state, whichforms a disulfide bond.
polar amino acid side groups possess separated
charges like those found on glutamine. Separated charges lead to dipole
interactions with other amino acids or with ligands binding to the protein.
Cysteine is a notable unique amino acid since in an
oxidizing extracel-lular environment, but not in the intracellular environment,
two cyste-ine residues in a protein can spontaneously oxidize to form a rather
stable disulfide bond (Fig. 6.3). In the isolated protein, this bond can be
reduced by the presence of an excess of a reducing reagent to regenerate
cysteines.
Related Topics