Chapter: Genetics and Molecular Biology: Protein Structure
Hydrogen Bonds and the Chelate Effect - Protein Structure
Hydrogen Bonds and the Chelate Effect
A hydrogen atom shared by two other atoms generates
a hydrogen bond. This sharing is energetically most important when the three
atoms are in a straight line and the atom to which the hydrogen is covalently
bonded, the hydrogen bond donor, possesses a partial negative charge
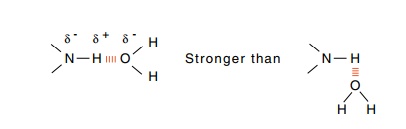
and the partner atom, a hydrogen bond acceptor,
also possesses a partial negative charge. Then the atoms may approach each
other quite closely and the electrostatic attractive forces and the dispersion
forces will be appreciable. Since the amide of the peptide bond can be a
hydrogen donor, and the carboxyl can be a hydrogen acceptor, proteins have a
potential for forming a great many hydrogen bonds. In addition, more than half
the side groups of the amino acids usually participate in hydrogen bonding.
A paradox is generated by the existence of hydrogen
bonds in pro-teins. Studies with model compounds show that a hydrogen bond to
water should be stronger than a hydrogen bond between amino acids. Why then
don’t proteins denature and make all their hydrogen bonds to water? A part of
the answer is the chelate effect. That is, two objects appear to bind to one
another far more strongly if something else holds them in the correct binding
positions than if their own attractive forces must correctly position the
objects. In a protein with a structure that holds amino acids in position, any
single bond between amino acids within the protein is entropically more
favorable than altering the structure of the protein and making the bond to
water. Another way of looking at this is that the formation of one hydrogen
bond holds other amino acids in position so that they may more easily form
hydrogen bonds themselves.
The chelate effect is important in understanding
many phenomena of molecular biology. A different example, explained more fully
later, concerns proteins. Much of the work required for two macromolecules to
bind to one another is correctly positioning and orienting them. Consider the
binding of a protein to DNA. If the protein and DNA have been correctly
positioned and oriented, then all of their interaction energy can go into
holding the two together. In the binding of a dimeric protein to DNA, once the
first subunit has bound, the second subunit is automatically positioned and
oriented correctly. Therefore the second subunit appears to have the larger
effect in binding the protein to DNA than the first one. Equivalently, the
dimer appears to bind more tightly than would be predicted by simply doubling
the ∆G of the binding reaction of the monomer.
Related Topics