Chapter: Genetics and Molecular Biology: Protein Structure
Salt Effects on Protein-DNA Interactions
Salt Effects on Protein-DNA Interactions
Many of the macromolecular interactions of interest in molecular biology involve the binding of proteins to DNA. Experiments show that often the affinity of a protein for DNA is sharply reduced as the concentration of NaCl or KCl in the buffer is increased. It might seem that the source of such behavior would be attractive forces between positive charges in the protein and the negatively charged phosphates of the DNA. The presence of high salt concentrations would then shield the attractive forces and weaken the binding by affecting both the association rate and the dissociation rate. Experimentally, however, the concentration of salts in the buffer primarily affects only the dissocia-tion rates!
Consider the binding of a protein to DNA. Before
binding of the protein, positively charged ions must reside near the negatively
charged phosphate groups. Some of these will be displaced as the protein binds,
and this displacement, even though it is not the breaking of a covalent bond,
must be considered in the overall binding reaction. Let us con-sider these to
be sodium ions. The reaction can be written
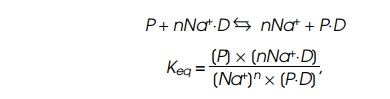
where P is protein concentration, D is DNA concentration, and an effective
number n of Na+ ions are displaced in the binding reaction. The fact
that n is often greater than five
makes the binding affinity sharply dependent upon salt concentration. The value
of n can be easily extracted from
experimental data by plotting the log of Kd
against the log of the salt concentration. Many regulatory proteins appear to
dis-place a net of four to ten ions as they bind.
The
physical basis for the ion strength dependence is straightforward. The
dissociation of a protein from DNA would require the nearly simultaneous and
precise binding of the n sodium ions
to their former positions on the DNA. The higher the concentration of sodium
ions in the solution, the more easily this may be accomplished.
The above
explanation can also be couched in thermodynamic terms. The entropic
contribution of the sodium ions to the binding-dissociation reaction is large.
Before the binding of the protein, the ions are localized near the backbone of
the DNA. Upon the binding of the protein, these ions are freed, with a
substantial entropy increase. The greater the sodium concentration in the
buffer, the smaller the entropy increase upon protein binding and therefore the
weaker the protein binding. Loosely bound, and therefore poorly localized, ions
do not make a substantial contribution to the changes in binding as the buffer
compo-sition is changed because their entropies do not change substantially
during the course of the reaction.
Related Topics