Chapter: Genetics and Molecular Biology: Protein Structure
Hydrophobic Forces - Protein Structure
Hydrophobic Forces
The structures of the many proteins that have been
determined by X-ray diffraction and nuclear magnetic resonance reveal that, in
general, the polar and charged amino acids tend to be found on the surface and
the aliphatic amino acids tend to be found in the interior. Hydrophobic
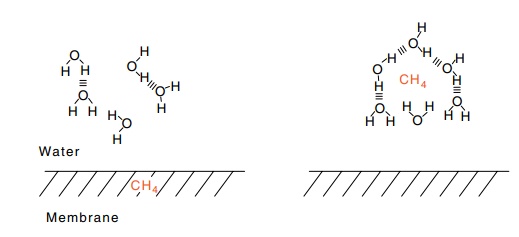
Figure
6.9 The creation of a water cage
around a hydrocarbon in water, whenit moves from membrane into water.
forces make aliphatic amino acids try to escape
from a water environ-ment and to cluster together in the center of a protein
away from water.
The precise definition of hydrophobic force and
methods of its measurement are currently under rapid development. One way of
considering the phenomenon begins by considering the energy and entropy change
in moving a neutral, nonpolar amino acid out of the interior of a protein and
into the surrounding water (Fig. 6.9). The entry of a hydrocarbon into water
facilitates the formation of structured cages of water molecules around the
hydrocarbon molecule. These surround the hydrocarbon but do not significantly
interact with it. The energy of formation of these structures actually favors
their generation, but the translational and rotational entropy loss required to
form the struc-tured water cages inhibits their production. From considerations
at this level, we cannot deduce the magnitude of the effects. Those are
deter-mined by measuring the relative solubility of different hydrocarbons in
water and organic solvents at various temperatures. The results show that the
state of the system in which these cages are absent, that is, with the nonpolar
amino acids in the interior of the protein, is more probable than the state in
which they are present on the protein’s surface.
Hydrophobic forces can be expected to be strongest
at some interme-diate temperature between freezing and boiling. Near freezing
tempera-tures, the water throughout the solution becomes more structured, and
thus there is little difference between the status of a water molecule in
solution or a water molecule in a cage around a hydrophobic group.
Alternatively, at high temperatures, little of the water around a hydro-phobic
group can be be structured. It is melted out of structure. The difference
between water around a hydrophobic group and water else-where in the solution
is maximized at some intermediate temperature. As this difference is important
to protein structure, some proteins possess maximum stability at intermediate
temperatures. A few are actually denatured upon cooling. A more common
manifestation of the hydrophobic forces is the fact that some polymeric
structures are destabilized by cooling and depolymerize
because the hydrophobic forces holding them together are weaker at lower
temperatures.
Related Topics