Chapter: Genetics and Molecular Biology: Protein Structure
Peptide Bond - Protein Structure
The Peptide Bond
A peptide bond links successive amino acids in a
polypeptide chain. The mere linking of amino acids to form a polypeptide chain,
however, is insufficient to ensure that the joined amino acids will adopt a
particular three-dimensional structure. The peptide bond possesses two
extraor-dinarily important properties that facilitate folding of a polypeptide
into a particular structure.
First, as a consequence of the partial double-bond
character of the peptide bond between the carbonyl carbon and nitrogen, the
unit
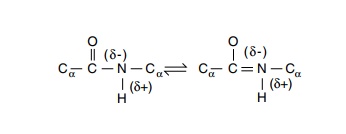
bounded by the alpha carbon atoms of two successive
amino acids is constrained to lie in a plane. Therefore, energy need not be
consumed from other interactions to generate the “proper” orientation about the
C-N bond in each amino acid. Rotation is possible about each of the two peptide
backbone bonds from the Cα atom of
each amino acid (Fig. 6.4). Angles of rotation about these two bonds are called
φ and ψ, and
their specification for each of the amino acids in a polypeptide completely
describes the path of the polypeptide backbone. Of course, the side chains of
the amino acids are free to rotate and may adopt a number of conformations so
that the φ and ψ angles do not completely specify the structure of a protein.
The second consequence of the peptide bond is that
the amide hydrogen from one amino acid may be shared with the carbonyl oxygen
from another amino acid in a hydrogen bond. Since each amino acid in
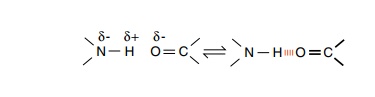
a polypeptide chain possesses both a hydrogen bond
donor and an acceptor, many hydrogen bonds may be formed, and in fact are
formed
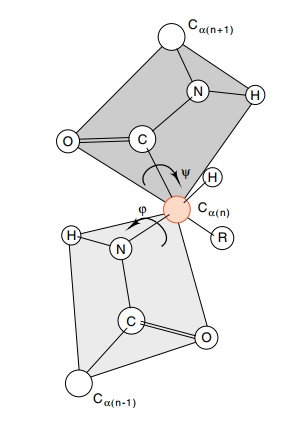
Figure 6.4 Two amino acid unitsin a polypeptide chain illustrating the planar structure of the peptide bond, the two degrees of rotational freedom for each amino acid unit in a polypeptide, and the angles ϕ and ψ.
in a polypeptide. Due to their positions on the
amino acids these bonds have to be between different amino acids in the
protein. Therefore they provide many stabilizing and structure-forming
interactions. Although the individual hydrogen bonds are weak, the large number
that can form in a protein contributes substantially to maintaining the
three-dimen-sional structure of a protein.
Related Topics