Chemistry - Structure of the functional group of alcohol | 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Structure of the functional group of alcohol
Structure of the functional group of alcohol.
The structure of -O-H group which is attached to a sp3
hybridised carbon is similar to the structure of -O-H group attached to a hydrogen
in water. i.e., ‘V’ shaped. In such alcohols, one of the sp3
hybridised orbital of oxygen linearly overlap with the sp3
hybridised orbital of carbon to form a C- O, ' σ ' bond and another sp3 hybridised orbital linearly
overlap with 1s orbital of hydrogen to form a O-H ' σ' bond.
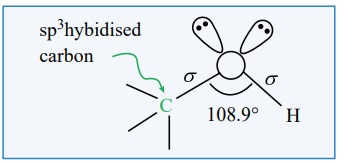
The remaining two sp3 hybridised orbitals of oxygen are
occupied by two lone pairs of electrons. Due to the lone pair – lone pair
repulsion, the C-O-H bond angle in methanol is reduced to 108.9° from the
regular tetrahedral bond angle of 109.5° .
Related Topics