Chemistry - Preparation of alcohols | 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Preparation of alcohols
Preparation of alcohols:
We have already learnt that the nucleophilic substitution reactions of
alkyl halides with dilute alkali, conversion of alkenes to alcohols by
hydration and the preparation of alcohols using Grignard reagent in XI
standard. These reactions are summarised below.
1. From Alkyl halides:
Alkyl halides on heating with dilute aqueous NaOH gives alcohols. Primary alkyl halides undergo substitution by SN2
reaction. Secondary and tertiary alkyl halides usually undergo nucleophilic
substitution by SN1 mechanism.
R-X + NaOH(aq) -----∆→ R-OH + NaX
If R =t-butyl, the reaction proceeds through the formation of t-butyl
carbocation
2. From alkenes:
Addition of water across the double bond of an alkene in
presence of concentrated sulphuric acid gives
alcohols. This addition reaction follows Markownikoff’s rule.
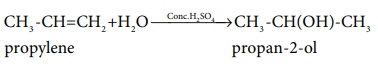
Example: CH3 -CH=CH2
+ H2O ---------Conc.H2SO4→ CH3
-CH(OH)-CH3
(propylene ---------Conc.H2SO4→ propan-2-ol )
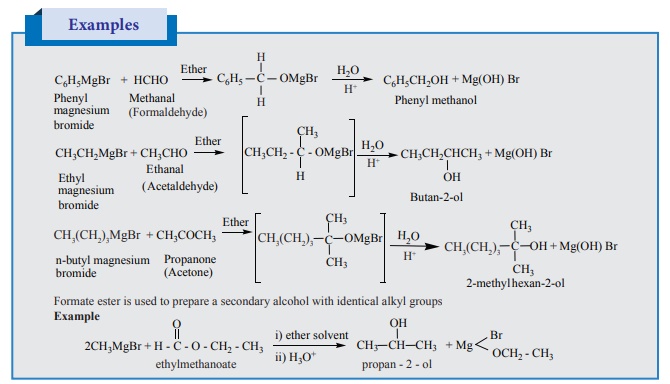
3. From Grignard reagent:
Nucleophilic addition of Grignard reagent to
aldehydes/ketones in presence of dry ether followed by the
acid hydrolysis gives alcohols. Formaldehyde gives primary alcohol and other
aldehydes give secondary alcohols. Ketones give tertiary alcohols.
Examples
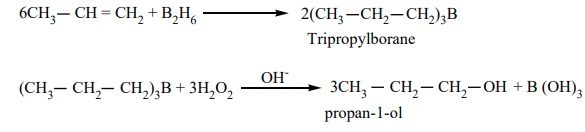
4. Hydroboration:
Diborane reacts with an alkene to form trialkyl borane which on treatment with H2O2 in presence of NaOH gives an alcohol. (Refer reactions of diborane) The overall reaction is hydration of an alkene. This reaction yields an anti-Markownikoff's product.
6CH3 -- CH = CH2 + B2H6 ------→ 2(CH3 --CH2--CH2)3B
(Tripropylborane)
(CH3 -- CH2 -- CH2)3B + 3H2O2
---OH-→ 3CH3
--CH2 --CH2 OH (propan-1-ol) + B (OH)3
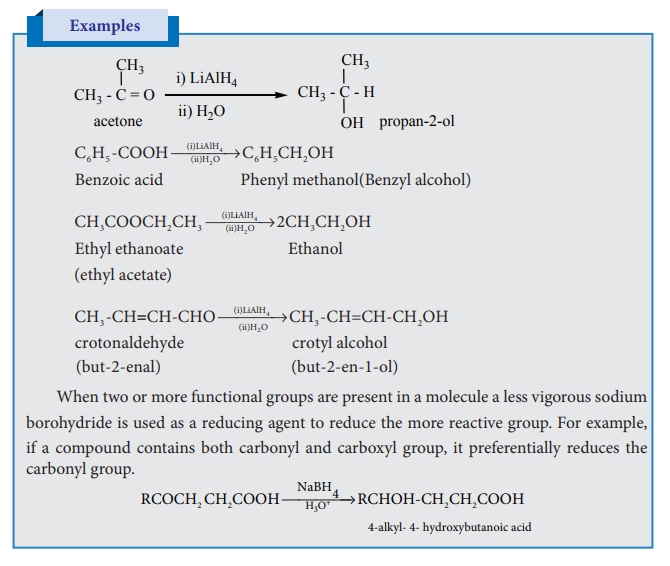
5. Reduction of carbonyl compounds:
Reduction of aldehydes/ketones with LiAlH4 in the presence of
solvents like THF (Tetrahydrofuran) followed by hydrolysis gives alcohols.
Unlike other reducing agents such as Raney Ni, Na-Hg/H2O, the
lithium aluminium hydride does not reduce the carbon–carbon double bond present in unsaturated carbonyl compound and
hence it is a best reagent to prepare unsaturated alcohols.
Examples
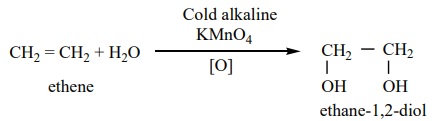
Preparation of glycol
We have already learnt that the hydroxylation of ethylene using cold
alkaline solution of potassium permanganate (Baeyer’s reagent) gives ethylene
glycol.
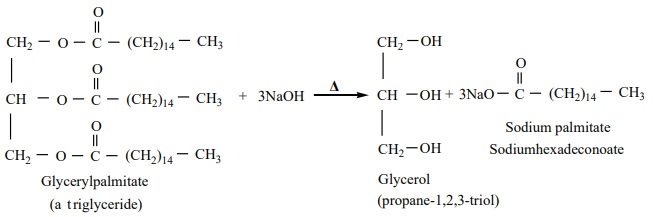
Preparation of glycerol
Glycerol occurs in many natural fats and it is also found in long chain
fatty acids in the form of glyceryl esters (Triglycerides). The alkaline
hydrolysis of these fats gives glycerol and the reaction is known as
saponification.
Evaluate Yourself?
1. Suggest a suitable carbonyl compound for the
preparation of pent-2-en-1-ol using LiAlH4 .
2. 2-methylpropene
-----H2SO4 /H2O →?
3. How will you prepare the following
using Grignard reagent. i) t-butyl alcohol ii) allyl alcohol
Methods to differentiate primary, secondary and tertiary alcohols.
The following tests are used to distinguish between 1°, 2° and 3°
alcohols.
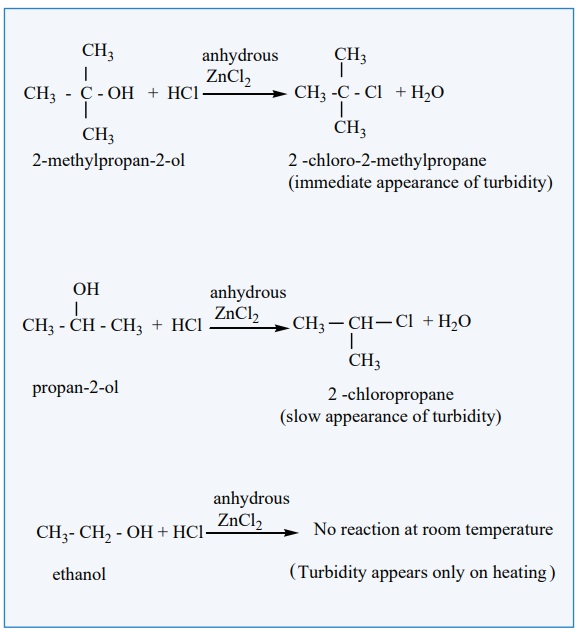
a) Lucas test:
When alcohols are treated with Lucas agent (a mixture of concentrated
HCl and anhydrous ZnCl2 ) at room temperature, tertiary alcohols
react immediately to form a turbidity due to the formation of alkyl chloride
which is insoluble in the medium. Secondary alcohols react within 10 minutes to
form a turbidity of alkyl chloride where primary alcohols do not react at room
temperature.
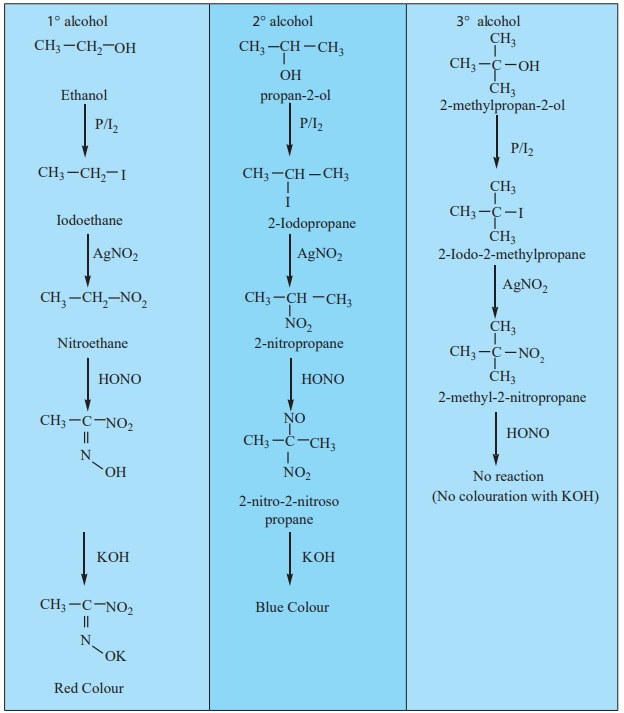
b) Victor Meyer’s test:
This test is based on the behaviour of the different nitro alkanes
formed by the three types of alcohols with nitrous acid and it consists of the
following steps.
i) Alcohols are converted into alkyl iodide by treating it with I2
/P .
ii) Alkyl iodide so formed is then treated with AgNO2 to form
nitro alkanes.
iii) Nitro alkanes are finally treated with HNO2 (mixture of
NaNO2 / HCl ) and the resultant solution is made alkaline with KOH.
Result:
• Primary alcohol gives red colour
• Secondary alcohol gives blue colour.
• No colouration will be observed in case of tertiary alcohol.
Related Topics