Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
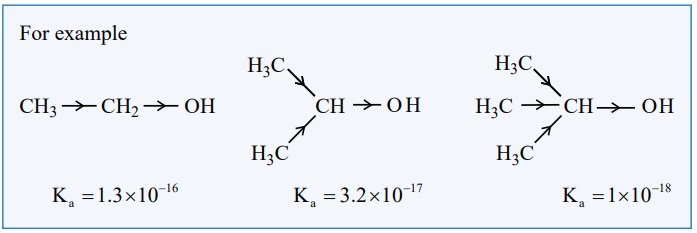
Comparison of acidity of 1┬║, 2┬║and 3┬║ alcohols
Comparison of acidity of 1┬║, 2┬║and 3┬║ alcohols
The acidic nature of the alcohol is due to the polar nature of O ŌĆōH
bond. When an electron withdrawing -I groups such as -Cl, - F etcŌĆ” is attached
to the carbon bearing the OH group, it withdraws the electron density towards
itself and thereby facilitating the proton donation. In contrast, the electron
releasing group such as alkyl group increases the electron density on oxygen
and decreases the polar nature of O ŌĆō H bond, Hence it results in the decrease
in acidity. on moving from primary to secondary and tertiary alcohols, the
number of alkyl groups which attached to the carbon bearing -OH group increases,
which results in the following order of acidity.
1┬║ alcohol > 2┬║ alcohol > 3┬║ alcohol
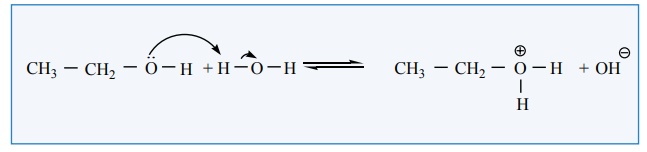
Alcohols can also act as a Bronsted bases. It is due to the presence of
unshared electron pairs on oxygen which make them proton acceptors.
Related Topics