Hydroxy Compounds and Ethers | Chemistry - Choose the correct answer | 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Choose the correct answer
Hydroxy Compounds and Ethers | Chemistry
EVALUATION
Choose the correct answer:
1. An alcohol (x) gives blue colour in Victormeyer’s test and 3.7g of X when treated with metallic sodium liberates 560 mL of hydrogen at 273 K and 1 atm pressure what will be the possible structure of X?
a) CH3 CH (OH) CH2CH3
b) CH3 – CH (OH) – CH3
b) CH3 C (OH) (CH3)2
d) CH3- CH2 –CH (OH) – CH2 – CH3
Solution:
2 R - OH + 2Na→ 2 RONa + H2↑2 moles of alcohol gives 1 mole of H2 which occupies 22.4L at 273K and 1 atm
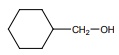
∴ number of moles of alcohol = ( 2 moles of R - OH / 22.4 L of H2 ) × 560 mL
= 0.05 moles
∴ no. of moles = mass / molar mass
⇒ molar mass = 3.7/0.05 = 74 g mol−1
General formula for R - OH Cn H2n+1 - OH
∴ n(12) + (2n+1) (1) + 16+1=74
14n = 74 – 18
14n = 56
∴ n = 56/14 = 4
The 2 alcohol which contains 4 carbon is CH2 CH(OH)CH2CH3
Option (a)
2. Which of the following compounds on reaction with methyl magnesium bromide will give tertiary alcohol.
a) benzaldehyde
b) propanoic acid
c) methyl propanoate
d) acetaldehyde
Solution:
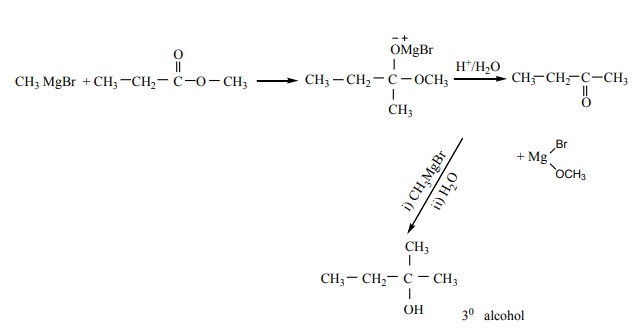
Option (c)
3. 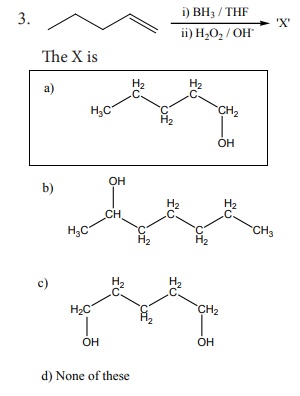
[Option: a]
Solution:
Hydro boration – Anti markownikoft product i.e., CH3 - CH2 - CH – CH2 – CH2 - OH
Option (a)
4. In the reaction sequence, Ethene ----HOCl→A ---X→ ethan -1, 2 - diol . A and X respectively are
a) Chloroethane and NaOH
b) ethanol and H2 SO4
c) 2 – chloroethan -1-ol and NaHCO3
d) ethanol and H2O
Solution:
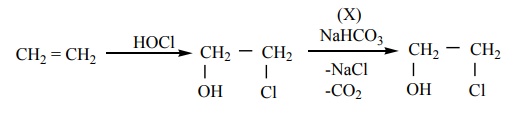
Solution: Option (c)
5. Which one of the following is the strongest acid
a) 2 - nitrophenol
b) 4 – chlorophenol
c) 4 – nitrophenol
d) 3 – nitrophenol
Solution: Option (c)
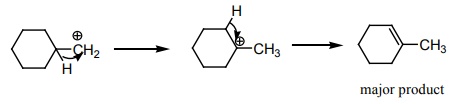
6.  on treatment with Con H2 SO4 , predominately gives
on treatment with Con H2 SO4 , predominately gives
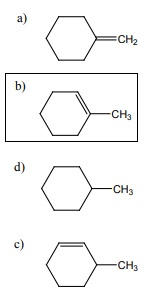
[Option: b]
Solution: Option (b) saytzeff rule
7. Carbolic acid is
a) Phenol
b) Picric acid
d) benzoic acid
d) phenylacetic acid
Solution: Option (a)
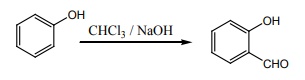
8. Which one of the following will react with phenol to give salicyladehyde after hydrolysis.
a) Dichloro methane
b) trichloroethane
c) trichloro methane
d) CO2
Solution: Riemer – Tiemann reaction (option (c))
9. (CH3 )3 - C - CH(OH) CH3 -----Con H2 SO4→ X (major product)
a) (CH3 )3 CCH = CH2
b) (CH3 )2 C = C (CH3)2
c) CH2 = C(CH3 )CH2 - CH2 - CH3
d) CH2 = C (CH3 ) - CH2 - CH2 - CH3
Solution:
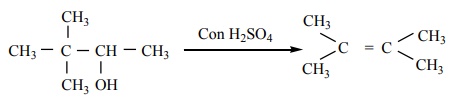
Option (b)
10. The correct IUPAC name of the compound, 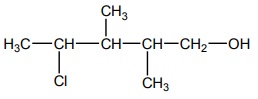
a) 4 – chloro – 2,3 – dimethyl pentan – 1-ol
b) 2,3 – dimethyl – 4- chloropentan -1-ol
c) 2,3,4 – trimethyl – 4- chlorobutan -1-ol
d) 4 – chloro – 2,3,4 – trimethyl pentan – 1-ol
Solution: Option (a)
11. Assertion : Phenol is more acidic than ethanol
Reason: Phenoxide ion is resonance stabilized
a) both assertion and reason are true and reason is the correct explanation of assertion.
b) both assertion and reason are true but reason is not the correct explanation of assertion.
c) assertion is true but reason is false
d) both assertion and reason are false.
Solution: Option (a)
12. In the reaction Ethanol 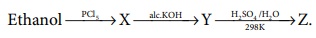 . The ‘Z’ is
. The ‘Z’ is
a) ethane
b) ethoxyethane
c) ethylbisulphite
d) ethanol
Solution:
CH3 - CH2 - OH ---PCl5→ CH3 - CH2 - Cl --ale.KOH→CH2 = CH2 --H2SO4/H2O→ CH3 - CH2 – OH
(Z) ethanol
Solution: Option (d)
13. The reaction
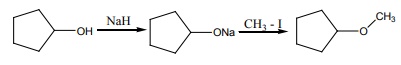
Can be classified as
a) dehydration
b) Williamson alcoholsynthesis
c) Williamson ether synthesis
d) dehydrogenation of alcohol
Solution:
Cyclic alcohol → sodium cyclic alkoxide → williamson ether synthesis
option (c)
14. Isopropyl benzene on air oxidation in the presence of dilute acid gives
a) C6 H5COOH
b) C6 H5COCH3
c) C6 H5 COC6 H5
d) C6 H5 - OH
Solution:
Option (d) phenol
15. Assertion : Phenol is more reactive than benzene towards electrophilic substitution reaction
Reason : In the case of phenol, the intermediate arenium ion is more stabilized by resonance.
a) if both assertion and reason are true and reason is the correct explanation of assertion.
b) if both assertion and reason are true but reason is not the correct explanation of assertion.
c) assertion is true but reason is false
d) both assertion and reason are false.
Solution: Option (a)
16. HO CH2 CH2 – OH on heating with periodic acid gives
a) methanoic acid
b) Glyoxal
c) methanal
d) CO2
Solution: Option (c)
17. Which of the following compound can be used as artifreeze in automobile radiators?
a) methanol
b) ethanol
c) Neopentyl alcohol
d) ethan -1, 2-diol
Solution: Option (d)
18. The reactions
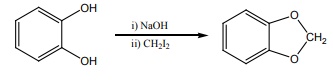
is an example of
a) Wurtz reaction
b) cyclic reaction
c) Williamson reaction
d) Kolbe reactions
Solution: Option (c)
19. One mole of an organic compound (A) with the formula C3 H8O reacts completely with two moles of HI to form X and Y. When Y is boiled with aqueous alkali it forms Z. Z answers the iodoform test. The compound (A) is
a) propan – 2-ol
b) propan -1-ol
c) ethoxy ethane
d) methoxy ehane
Solution:
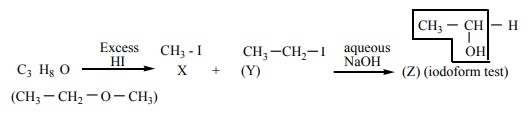
Option (d)
20. Among the following ethers which one will produce methyl alcohol on treatment with hot HI?
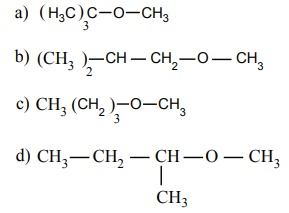
[Option: a]
Solution:
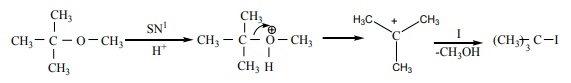
Option (a)
21. Williamson synthesis of preparing dimethyl ether is a / an /
a) SN1 reactions
b) SN2 reaction
c) electrophilic addition
d) electrophilic substitution
Solution: Option (b) SN2 reaction
22. On reacting with neutral ferric chloride, phenol gives
a) red colour
b) violet colour
c) dark green colour
d) no colouration.
Solution: Violet color option (b)
Related Topics