Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Acidity of Phenol
Acidity of Phenol
Phenol is more acidic than aliphatic alcohols. Unlike alcohols it reacts
with bases like sodium hydroxide to form sodium phenoxide. This explains the
acidic behaviour of phenol.let us consider the aqueous solution of phenol in
which the following equilibrium exists.

Ka
value for the above equilibrium is 1Ă—10-10 at 25ÂşC . This Ka value indicates that it is more acidic
than aliphatic alcohols. This increased acidic behaviour can be explained on
the basis of the stability of phenoxide ion. We have already learnt in XI
standard that the phenoxide is more stabilised by resonance than phenol.
In substituted phenols, the electron withdrawing groups such as -NO2
,-Cl enhances the acidic nature of phenol especially when they are present at
ortho and para positions. In such cases,there is a possibility for the extended
delocalisation of negative charge on the phenoxide ion. On the other hand the
alkyl substitued phenols show a decreased acidity due to the electron releasing
+I effect of alkyl group.
Table:
pKaValues of some alcohols and phenols
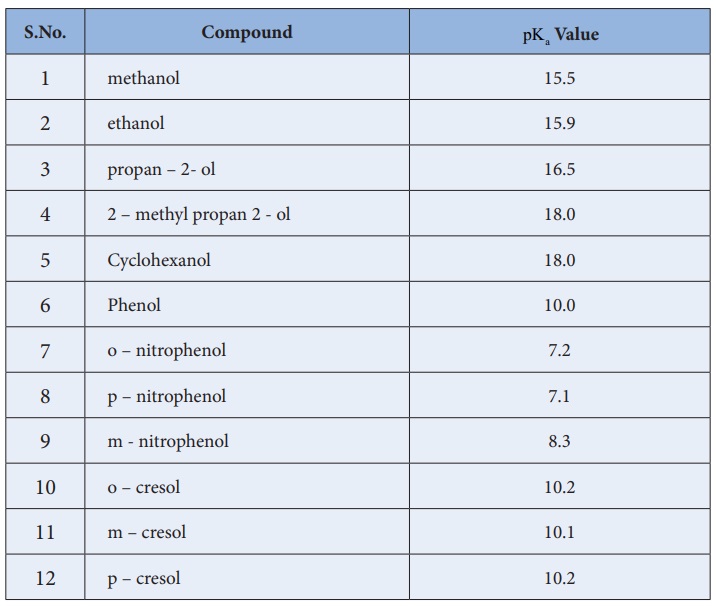
S.No. : Compound
- pKa Value
1. methanol : 15.5
2. ethanol : 15.9
3. propan – 2- ol : 16.5
4. 4 2 – methyl propan 2 - ol : 18.0
5. Cyclohexanol : 18.0
6. Phenol : 10.0
7. o – nitrophenol : 7.2
8. p – nitrophenol : 7.1
9. m - nitrophenol : 8.3
10. o – cresol : 10.2
11. m – cresol : 10.1
12. p – cresol : 10.2
Related Topics