Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Physical and Chemical Properties of alcohols
Properties of alcohols
Physical properties
i. Lower alcohols are colourless liquids and the
higher members are waxy solids.
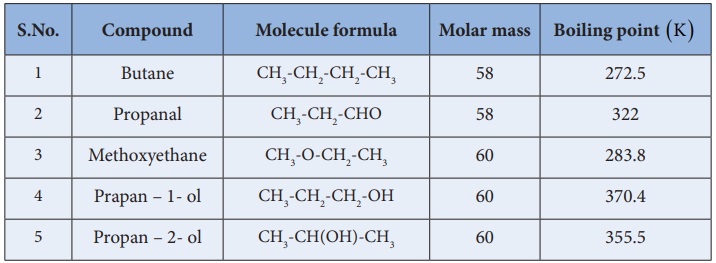
ii. They have higher boiling points than the
corresponding other organic compounds such as alkanes, aldehydes, ethers etc.,
this is due to the presence of intermolecular hydrogen bonding present in
alcohols.
iii. Among isomeric alcohols primary alcohols have higher boiling point
and the tertiary alcohols have lower boiling points.
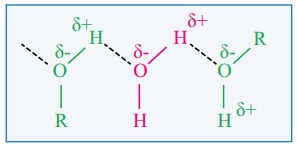
iv. The lower members are highly soluble in water due to the formation
of intermolecular hydrogen bonding with water.
Table : Boiling point of alcohols in comparision with other organic compounds.
Chemical properties of alcohols
Nucleophilic substitution reactions of alcohols
Alcohol has a strong basic leaving group (OH–). So, –OH group is first
converted into −+OH2 group by
adding an acid. The −+OH2 group in the protonated alcohol can be easily displaced
by a nucleophile such as Br- to give alkyl halides.
Example:
Alcohols undergo nucleophilic substitution reaction with hydro halic acids to form alkyl halides. In case of tertiary
alcohols heating is required.
Alkyl halide formation from primary alcohols follow SN 2 mechanism
Example
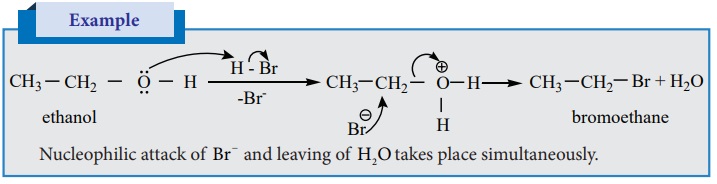
Nucleophilic attack of Br− and leaving of H2O takes place
simultaneously.
Alkyl halide formation of tertiary alcohols follow SN1mechanism.
Example
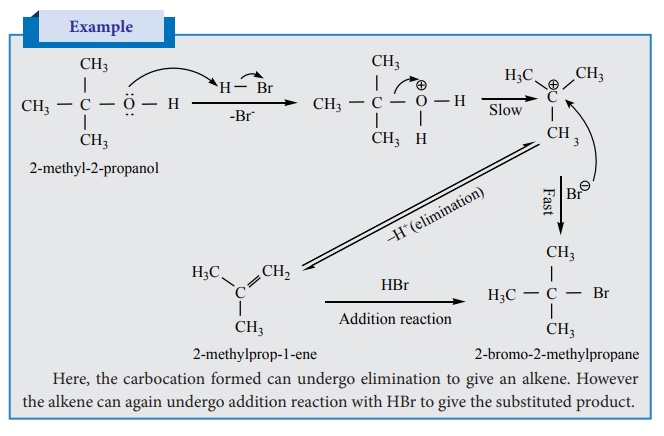
Here, the carbocation formed can undergo elimination to
give an alkene. However the alkene can again undergo addition reaction with HBr
to give the substituted product.
Conversion of alcohol into alkyl halides: Other methods
Alcohols can also be converted into an alkyl halides using PCl3
, PBr3 .
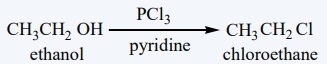
Mechanism
: SN 2
reaction on phosphorous tri chloride
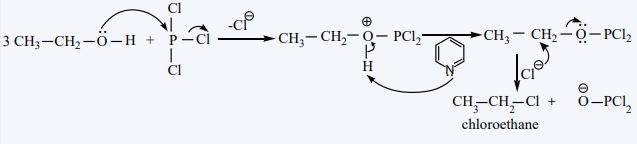
The conversion of an alcohol to alkyl halide can also be effected using
thionyl chloride
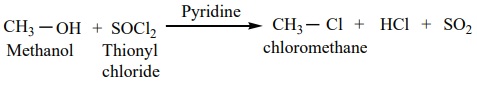
This reaction also follows theSN i mechanism in the presence of pyridine.
Elimination reactions of alcohols
When alcohols are heated with a suitable dehydrating agents like sulphuric acid, the H and OH present in the adjacent carbons of alcohols are lost, and it results in the formation of a carbon – carbon double bond. Phosphoric acid, anhydrous ZnCl2 , alumina etc., can also be used as dehydrating agents.
Example
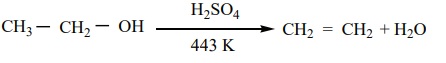
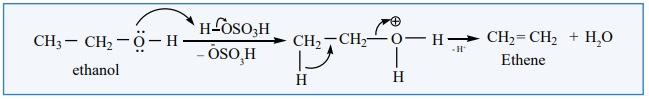
Mechanism
Primary alcohols undergo dehydration by E2 mechanism
Tertiary alcohols undergo dehydration by E1 mechanism. It
involves the formation of a carbocation.
Protonation of alcohol
Step 1 :
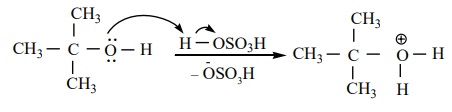
Step 2 : Dissociation of oxonium ion to form a
carbocation.
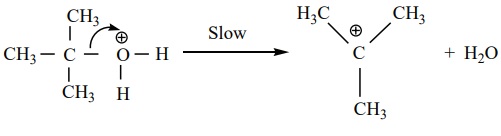
Step 3 : Deprotonation of carbocation to form an
alkene
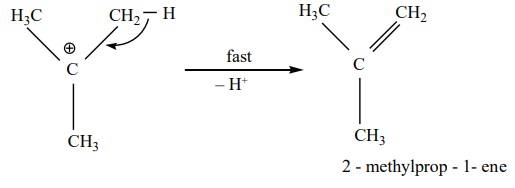
Order of reactivity:
The relative reactivities of alcohols in the dehydration reaction
follows the
order primary < secondary < tertiary
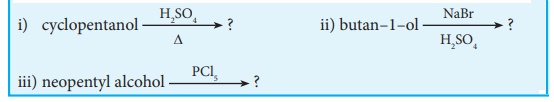
Evaluate yourself
Identify the products in the following reactions. Write
their IUPAC names and mention the mechanism involved in the reactions.
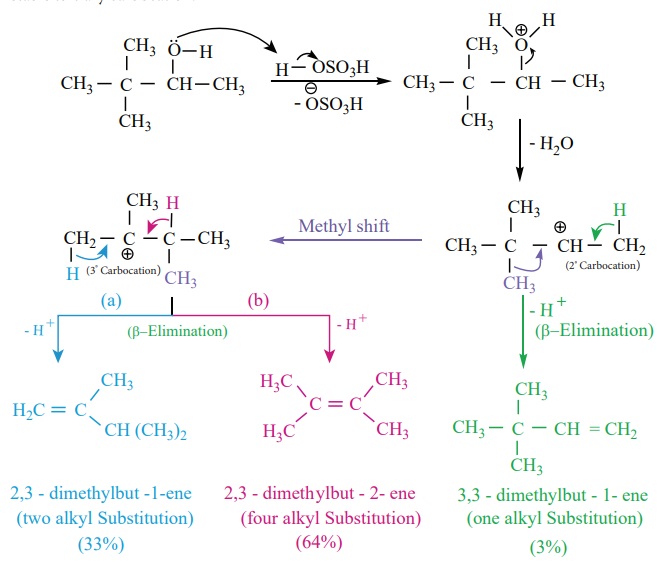
Saytzeff’s rule
During intramolecular dehydration, if there is a possibility to form a
carbon – carbon double bond at different locations, the preferred location is
the one that gives the more (highly) substituted alkene i.e., the stable
alkene.
For example, the dehydration of 3,3 – dimethyl – 2- butanol gives a
mixture of alkenes. The secondary carbocation formed in this reaction undergoes
rearrangement to form a more stable tertiary carbocation.
Evaluate yourself : What is the major product obtained
when 2,3 – dimethyl pentan -3 – ol is
heated in the presence of H2SO4 .
Oxidation of alcohols
The important reactions of alcohols are their oxidation to give carbonyl
compounds. The commonly used oxidising agent is acidified sodiumdichromate.
Oxidation of primary alcohols give an aldehyde which on further oxidation gives
the carboxylic acids. To stop the oxidation reaction at the aldehyde / ketone
stage, pyridinium chlorochromate (PCC) is used as an oxidising agent.
Example
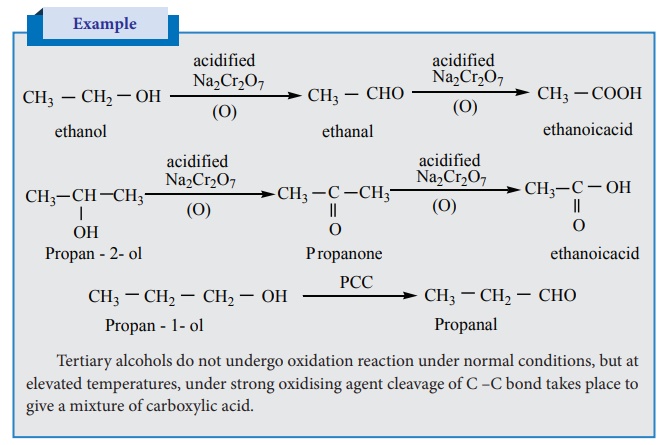
Tertiary alcohols do not undergo oxidation reaction under
normal conditions, but at elevated temperatures, under strong oxidising agent
cleavage of C –C bond takes place to give a mixture of carboxylic acid.
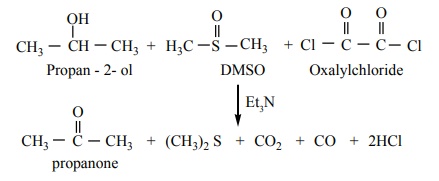
Swern oxidation
In this method, dimethyl sulfoxide (DMSO) is used as the oxidising
agent, which converts alcohols to ketones / aldehydes.
In this method an alcohol is treated with DMSO and oxalyl chloride
followed by the addition of triethylamine.
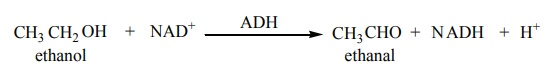
Biological oxidation
The fermentation of the food consumed by an animal produces alcohol. To
detoxify the alcohol, the liver produces an enzyme called alcohol dehydrogenase
(ADH). Nicotinamide adenine dinucleotide (NAD) present in the animals act as an
oxidising agent and ADH catalyses the oxidation of toxic alcohols into
non-toxic aldehyde.
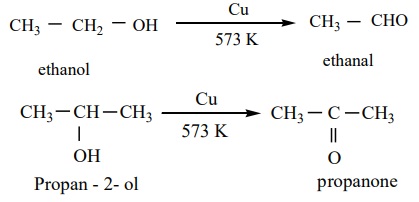
Catalytic dehydrogenation
When the vapours of a primary or a secondary alcohol are passed over heated
copper at 573K, dehydrogenation takes place to form aldehyde or ketone.
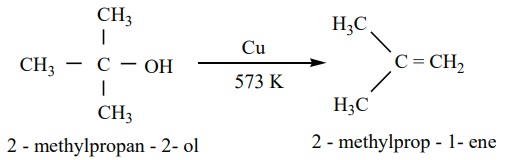
Tertiary alcohols undergo dehydration reaction to give alkenes.
Esterification
Alcohols react with carboxylic acids in the presence of an acid to give
esters
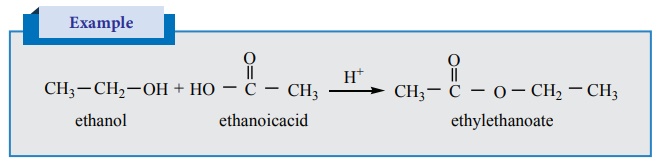
Reactions of Glycol
Ethylene glycol contains two primary alcoholic groups and it exhibits
the usual reactions of hydroxyl group. Like other primary alcohols, it reacts
with metallic sodium to form monosodium glycolate and disodium glycolate. The
hydroxyl groups can be converted to the halide groups by treating glycol with
halic acid (or with PCl5 / PCl3 / SOCl2.)
When ethylene glycol is treated with HI or P/I2, 1,2 –
diiodoethane is first formed which decomposes to give ethene.
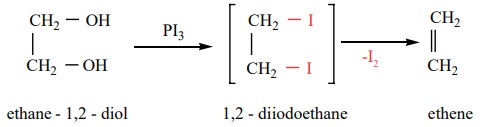
On heating with conc HNO3 in the presence of Con. H2SO4,
ethylene glycol forms dinitroglycol.
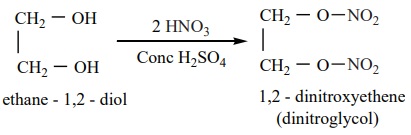
Dehydration reaction
Ethyleneglycol undergoes dehydration reaction under different conditions
to form different products.
1. When heated to 773K, it forms epoxides.
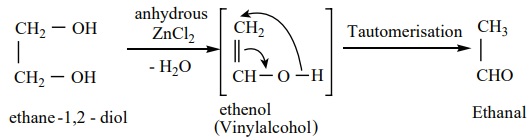
2. When heated with dilute sulphuric acid (or) anhydrous ZnCl2 under pressure in a sealed tube, it gives acetaldehyde.
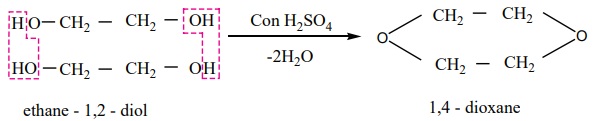
3. When distilled with Conc. H2 SO4 , glycol forms
dioxane
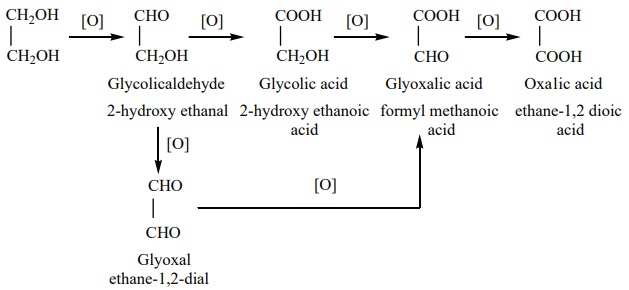
Oxidation of glycol
On oxidation, glycol gives a variety of products depending on the nature
of oxidizing agent and other reaction conditions.
i) When nitric acid (or) alkaline potassium permanganate is used as the
oxidizing agent, the following products are obtained.
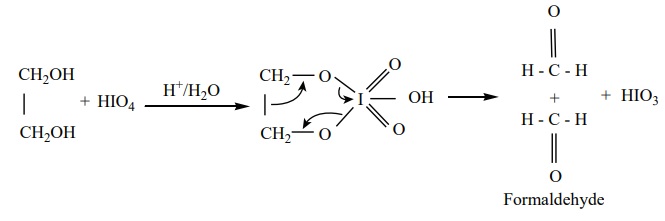
ii) Oxidation of glycol with periodic acid
Ethylene glycol on treatment with periodic acid gives formaldehyde. This
reaction is selective for vicinal 1,2 – diols and it proceeds through a cyclic
periodate ester intermediate.
Reaction of Glycerol
Nitration:
Glycerol
reacts with concentrated nitric acid in the presence of concentrated sulphuric acid to form TNG
(nitroglycerine).
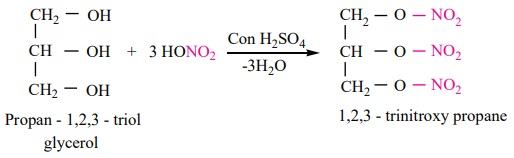
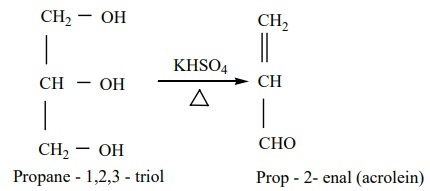
Dehydration
When glycerol is heated with dehydrating agents such as Con H2SO4
,KHSO4 etc….,
it undergoes dehydration to form acrolein.
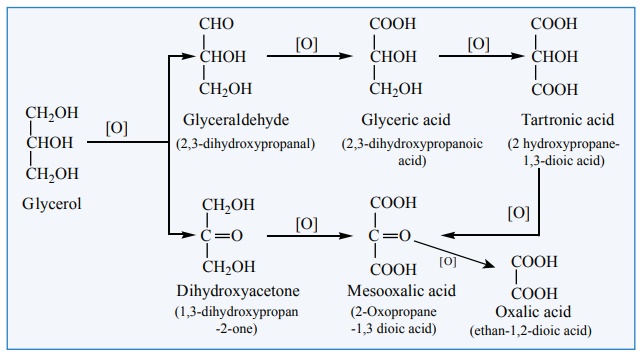
Oxidation
Glycerol can give rise to a variety of oxidation products depending on
the nature of the oxidising agent used for oxidation.
a) Oxidation of glycerol with dil. HNO3
gives glyceric acid and tartronic acid.
b) Oxidation of glycerol with Conc. HNO3
gives mainly glyceric acid.
c) Oxidation of glycerol with bismuth nitrate gives
as meso oxalic acid.
d) Oxidation of glycerol with Br2/H2O
(or) NaOBr (or) Fenton's reagent (FeSO4 + H2O2)
gives a mixture of glyceraldehyde and dihydroxy acetone(This mixture is named
as glycerose).
e) On oxidation with HIO4 or Lead tetra
acetate (LTA) it gives formaldehyde and formic acid.
f) Acidified KMnO4 oxidises glycerol into oxalicacid.
Related Topics