Classification, Preparation, Physical and Chemical Properties, Uses of phenol, Common electrophilic aromatic substitutions - Phenols | 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
Chapter: 12th Chemistry : UNIT 11 : Hydroxy Compounds and Ethers
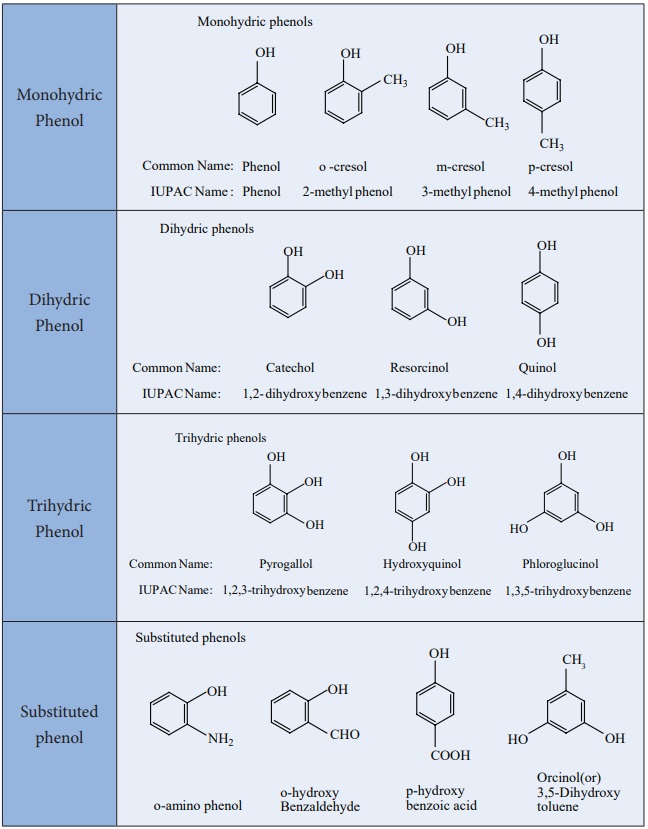
Phenols
Phenols:
Phenols are organic compounds in which a -OH group is directly attached
to a benzene ring. The carbon bearing the -OH group is sp2
hybridized.
Table: Classification of phenols
Preparation of Phenols
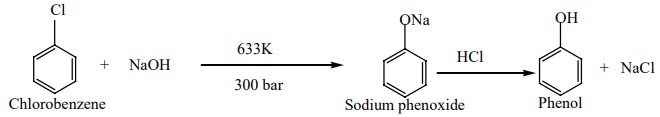
a) From
halo arenes(Dows process)
When Chlorobenzene is hydrolysed with 6-8% NaOH at 300 bar and 633K in a
closed vessel,sodium phenoxide is formed which on treatment with dilute HCl
gives phenol.
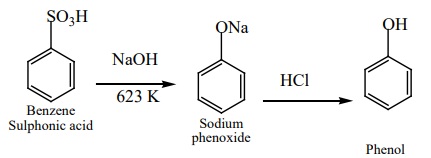
b) From
benzene sulphonic acid
Benzene is sulphonated with oleum and the benzene sulphonic acid so
formed is heated with molten NaOH at 623K gives sodium phenoxide which on
acidification gives phenol.
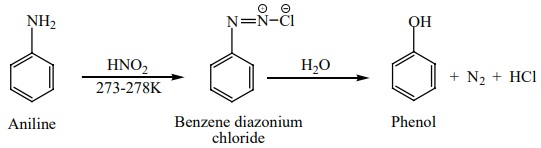
c) From
aniline
Aniline is diazotized with nitrous acid ( NaNO2 +HCl ) at
273-278K to give benzene diazonium chloride which on further treatment with hot
water in the presence of mineral acid gives phenol.
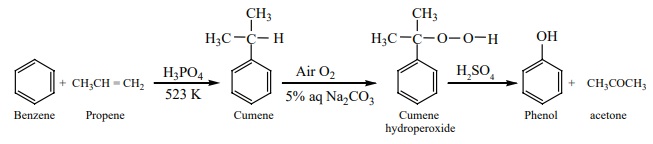
d) From
cumene
A mixture of benzene and propene is heated at 523K in a closed vessel in
presence of H3PO4 catalyst gives cumene (isopropylbenzene).
On passing air to a mixture of cumene and 5% aqueous sodium carbonate solution,
cumene hydro peroxide is formed by oxidation. It is treated with dilute acid to
get phenol and acetone. Acetone is also an important byproduct in this
reaction.
Physical Properties
Phenol is colourless, needle shaped crystal, hygroscopic, corrosive and
poisonous. It turns pink on exposure to air and light. The simplest phenols are
liquids or low melting solids, they have quite high boiling points. Phenol is
slightly soluble in water because of hydrogen bonding. However other
substituted phenols are essentially insoluble in water.
Chemical Properties:
Reactions involving -OH group.
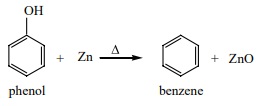
a) Reaction with Zn dust:
Phenol is converted to benzene on heating with zinc dust. In this
reaction the hydroxyl group which is attached to the aromatic ring is
eliminated.
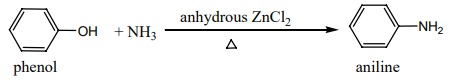
b) Reaction with ammonia:
Phenol on heating with ammonia in presence of anhydrous ZnCl2
gives aniline.
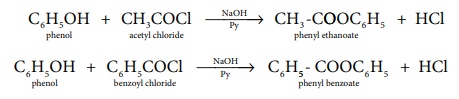
c) Formation of esters:
Schotten-Baumann reaction :
Phenol on treatment with acid chlorides gives esters. The acetylation
and benzoylation of phenol are called Schotten-Baumann reaction.
d) Formation of ethers:
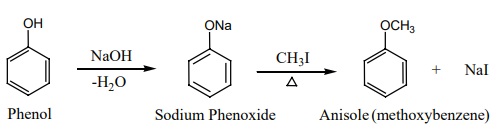
Williamson ether synthesis:
An alkaline solution of phenol reacts with alkyl halide to form phenyl
ethers. The alkyl halide undergoes nucleophilic substitution by the phenoxide
ion in the presence of alkali.
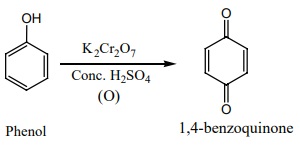
d) Oxidation:
Phenol undergoes oxidation with air or acidified K2Cr2O7
with conc. H2SO4 to form 1,4-benzoquinone.
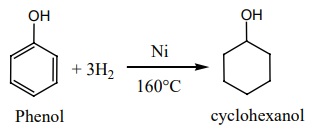
e) Reduction:
Phenol on catalytic hydrogenation gives cyclohexanol.
Reactions of benzene ring:
Electrophilic aromatic substitution:
We have already learnt in XI standard that the groups like 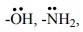 etc., which when directly attached to the benzene ring, activate the ring
towards electrophilic substitution reaction and direct the incoming
electrophile to occupy either the ortho or para position.
etc., which when directly attached to the benzene ring, activate the ring
towards electrophilic substitution reaction and direct the incoming
electrophile to occupy either the ortho or para position.
Common electrophilic aromatic substitutions are as follows:
i) Nitrosation:
Phenol can be readily nitrosoated at low temperature with HNO2
.
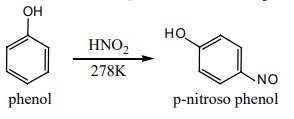
ii) Nitration:
Phenol can be nitrated using 20% nitric acid even at room temperature, a
mixture of ortho and para nitro phenols are formed.
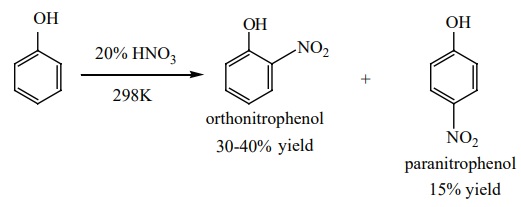
The ortho and para isomers are separated by steam distillation, as
o-nitro phenol is slightly soluble in water and more volatile due to intra
molecular hydrogen bonding, whereas p-nitro phenol is more soluble in water and
less volatile due to intermolecular hydrogen bonding.
Nitration with Conc. HNO3 +con.H2SO4
gives picric acid.
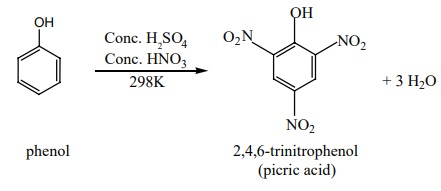
iii) Sulphonation:
Phenol reacts with con.H2 SO4 at 280K to form
o-phenol sulphonic acid as the major product. When the reaction is carried out
at 373K the major product is p-phenol sulphonic acid.
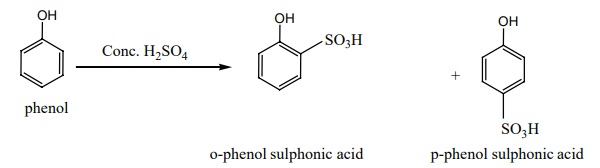
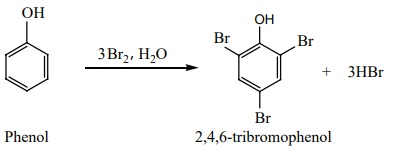
iv) Halogenation:
Phenol reacts with bromine water to give a white precipitate of 2,4,6-tri
bromo phenol.
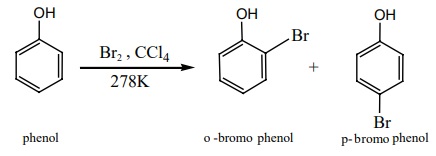
If the reaction is carried out in CS2 or CCl4 at
278K, a mixture of ortho and para bromo phenols are formed.
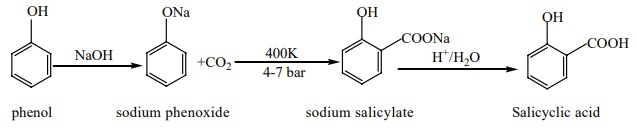
v) Kolbe’s (or) Kolbe’s Schmit reaction:
In this reaction, phenol is first converted into sodium phenoxide which
is more reactive than phenol towards electrophilic substitution reaction with
CO2 . Treatment of sodium phenoxide with CO2 at 400K, 4-7
bar pressure followed by acid hydrolysis gives salicylic acid.
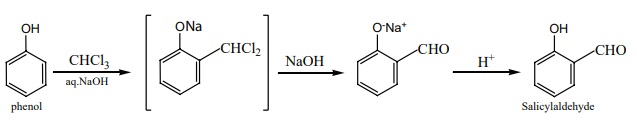
vi) Riemer – Tiemann Reaction:
On treating phenol with CHCl3 /NaOH, a -CHO group is
introduced at ortho position. This reaction proceeds through the formation of
substituted benzal chloride intermediate.
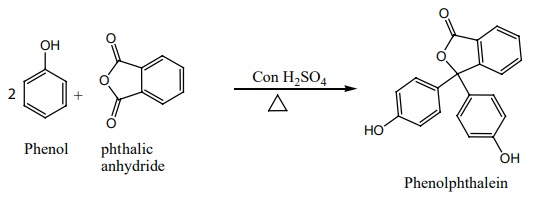
vii) Phthalein reaction:
On heating phenol with phthalic anhydride in presence of con.H2
SO4 , phenolphthalein is obtained.
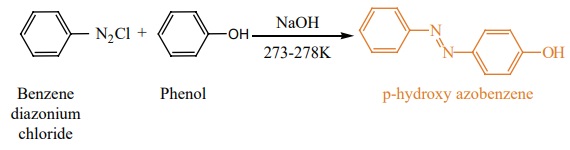
viii) Coupling reaction:
Phenol couples with benzene diazonium chloride in an alkaline solution
to form p-hydroxy azobenzene(a red orange dye).
Test to differentiate alcohol and phenols
i) Phenol react with benzene diazonium chloride to
form a red orange dye, but ethanol has no reaction with it.
ii) Phenol gives purple colouration with neutral
ferric chloride solution, alcohols do not give such coloration with FeCl3
.
iii) Phenol reacts with NaOH to give sodium phenoxide. Ethyl alcohol
does not react with NaOH .
Uses of phenol
1) About half of world production of phenol is used
for making phenol formaldehyde resin. (Bakelite).
2) Phenol is a starting material for the
preparation of
i) drugs such as phenacetin, Salol, aspirin, etc.
ii) phenolphthalein indicator.
iii) explosive like picric acid.
3) It is used as an antiseptic-carbolic lotion and carbolic soaps.
Evaluate Yourself
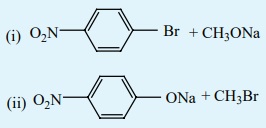
1. Which of the following set of reactants will give
1-methoxy-4-nitrobenzene.
2. what happens when m-cresol is treated with acidic
solution of sodium dichromate?
3. when phenol is treated with propan-2-ol in the presence
of HF, Friedel-Craft reaction takes place . Identify the products.
Related Topics