Chapter: Genetics and Molecular Biology: RNA Polymerase and RNA Initiation
Steps of the Initiation Process
Steps of the Initiation Process
The beginning state for initiation by RNA
polymerase is a polymerase molecule and a promoter free in solution, and the
end state is a polymerase molecule bound to DNA elongating an RNA chain. In
this state the DNA is partially melted so that base-pairing of ribonucleotides
to the template strand of the DNA can be used to determine the nucleotides to
be incorporated into the RNA. The initiation process that separates the two
states must be continuous, but it may well be approxi-mated as consisting of
discrete steps. Can any of these be detected and quantitated and, if so, can
measurement of the rates of proceeding from one such state to the next during
the initiation process provide useful information? Ultimately we would hope
that studies like these could explain the differences in activities between
promoters of different sequence as well as provide the information necessary to
design pro-moters with specific desired activities or properties.
The first biochemical characterizations of the
binding and initiation rates of RNA polymerase on promoters were performed on
highly active promoters of bacterial origin. Such a choice for a promoter is
natural because it maximizes the signal-to-noise ratio in the data. The data
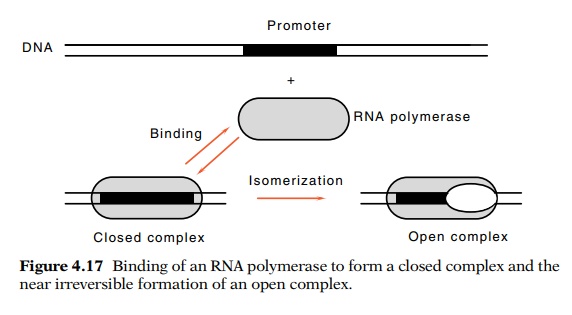
Figure
4.17 Binding of an RNA polymerase to
form a closed complex and thenear irreversible formation of an open complex.
obtained by Chamberlin with these promoters
indicated that the initia-tion process could be dissected into two steps: a
rapid step during which polymerase binds to DNA and a slower “isomerization”
step in which RNA polymerase shifts to an active form capable of immediately
initi-ating transcription (Fig. 4.17). Later studies on a wide variety of
pro-moters indicates that this approximation is generally useful. Some
promoters possess additional discernible steps in the initiation process.
It is natural to expect that the first step would
be the point for regulation of transcription. Since cells need to have
promoters of widely differing activity as well as promoters whose activities
can be controlled, it seems sensible that the binding step would vary among
promoters, and if auxiliary proteins are required for initiation at a promoter,
they would alter the binding rate. If the regulation of initiation by auxiliary
proteins occurred after the binding step, for example in the isomeriza-tion
step, then many polymerase molecules in cells would be nonpro-ductively bound
at promoters. This would be a waste of substantial numbers of polymerase
molecules. Apparently we lack important infor-mation, for nature actually has
it both ways. Some weak promoters have good binding of polymerase, but slow
isomerization, whereas the re-verse is true with others. Similarly, on some
regulated promoters regulation is at the binding step, and on others the
regulation occurs at the isomerization step or at a step subsequent to
isomerization.
Related Topics