Chapter: Clinical Anesthesiology: Regional Anesthesia & Pain Management: Spinal, Epidural & Caudal Blocks
Spinal Anesthesia
Spinal Anesthesia
Initially after injection, spinal anesthetic solutions inhibit
conduction in nerve roots as they course through the subarachnoid space. Over
time, the local anesthetic permeates the spinal cord and likely interacts with
other targets located therein. The spinal subarachnoid space extends from the
fora-men magnum to the S2 in adults and S3 in children. Injection of local
anesthetic below L1 in adults and L3 (below the termination of the conus
medullaris) in children helps to avoid direct trauma to the spi-nal cord.
Spinal anesthesia is sometimes referred to a subarachnoid block, and it occurs
as a result of an intrathecal injection.
Spinal Needles
Spinal needles are commercially available in
an array of sizes lengths, and bevel and tip designs (Figure
45–16). All should have a tightly fitting
removable stylet that completely occludes the lumen to avoid tracking
epithelial cells into the subarach-noid space. Broadly, they can be divided
into either sharp (cutting)-tipped or blunt-tipped needles. The Quincke needle
is a cutting needle with end injec-tion. The introduction of blunt tip
(pencil-point) needles has markedly decreased the incidence of postdural
puncture headache. The Whitacre and other pencil-point needles have rounded
points and
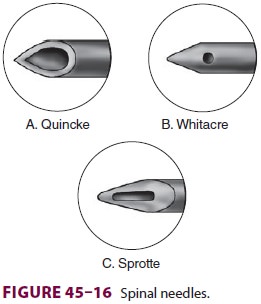
side injection. The Sprotte is a
side-injection needle with a long opening. It has the advantage of more
vigorous CSF flow compared with similar gauge needles. However, this can lead
to a failed block if the distal part of the opening is subarachnoid (with free
flow CSF), the proximal part is not past the dura, and the full dose of
medication is not delivered. In general, the smaller the gauge needle, the
lower the incidence of headache.
Spinal Catheters
Very small subarachnoid catheters are
currently no longer approved by the US Food and Drug Admin-istration. The
withdrawal of these catheters was prompted by their association with cauda
equina syndrome (CES). Larger catheters designed for epi-dural use are
associated with relatively high com-plication rates when placed subarachnoid;
however, they are frequently used for continuous spinal anes-thesia following
accidental dural puncture during performance of epidural anesthesia.
Specific Technique for Spinal Anesthesia
The midline, or paramedian, approaches, with
the patient positioned in the lateral decubitus, sitting, or prone positions,
can be used for spinal anesthe-sia. As previously discussed, the needle is advanced
from skin through the deeper structures until two “pops” are felt. The first is
penetration of the liga-mentum flavum, and the second is penetration of the
dura–arachnoid membrane. Successful dural puncture is confirmed by withdrawing
the stylet to verify free flow of CSF. With small-gauge needles (<25 g), aspiration may be
necessary to detect CSF. If free flow occurs initially, but CSF cannot be
aspi-rated after attaching the syringe, the needle likely will have moved.
Persistent paresthesias or pain with injection of drugs should alert the
clinician to with-draw and redirect the needle.
Factors Influencing Level of Spinal Block
Table 45–2lists
factors that have been shown toaffect the level of neural blockade following
spinal anesthesia. The most important determinants are baricity of the local
anesthetic solution, position of
the patient during and immediately after
injection, and drug dosage. In general, the larger the dosage or more cephalad
the site of injection, the more ceph-alad the level of anesthesia that will be
obtained. Moreover, migration of the local anesthetic cepha-lad in CSF depends
on its density relative to CSF (baricity). CSF has a specific gravity of
1.003–1.008 at 37°C. Table 45–3 lists the specific
gravity of anesthetic solutions. A hyperbaric solution of local anesthetic is
denser (heavier) than CSF, whereas a
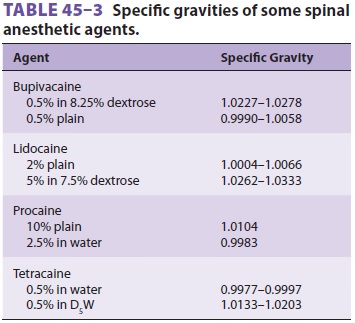
hypobaric solution is less dense (lighter)
than CSF. The local anesthetic solutions can be made hyper-baric by the
addition of glucose or hypobaric by the addition of sterile water or fentanyl.
Thus, with the patient in a head-down position, a hyperbaric solu-tion spreads
cephalad, and a hypobaric anesthetic solution moves caudad. A head-up position
causes a hyperbaric solution to settle caudad and a hypo-baric solution to
ascend cephalad. Similarly, when a patient remains in a lateral position, a
hyperbaric spinal solution will have a greater effect on the dependent (down)
side, whereas a hypobaric solu-tion will achieve a higher level on the
nondependent (up) side. An isobaric solution tends to remain at the level of
injection. Anesthetic agents are mixed with CSF (at least 1:1) to make their
solutions isobaric. Other factors affecting the level of neural blockade
include the level of injection and the patient’s height and vertebral column
anatomy. The direction of the needle bevel or injection port may also play a
role; higher levels of anesthesia are achieved if the injec-tion is directed cephalad
than if the point of injec-tion is oriented laterally or caudad.
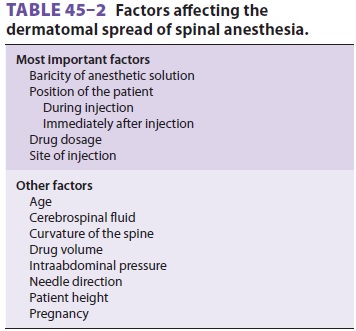
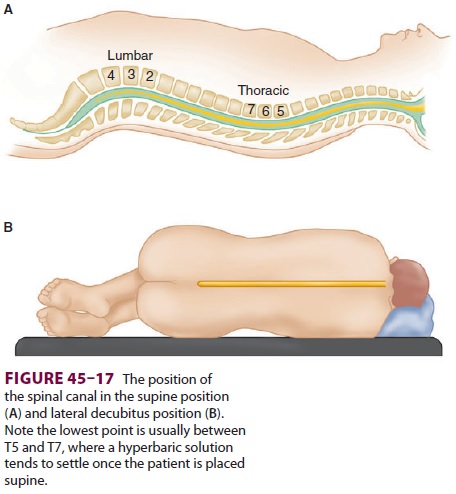
Hyperbaric solutions tend to move to the most
dependent area of the spine (normally T4–T8 in the supine position). With
normal spinal anatomy, the apex of the thoracolumbar curvature is T4 (Figure
45–17). In the supine position, this should limit
a hyperbaric solution to produce a level of anesthesia at or below T4. Abnormal
curvatures of the spine, such as scoliosis and kyphoscoliosis, have multiple
effects on spinal anesthesia. Placing the block becomes more difficult because
of the rotation and angulation of the vertebral bodies and spinous processes.
Finding the midline and the interlaminar space may be difficult. The paramedian
approach to lumbar puncture may be preferable in patients with severe scoliosis
and kyphoscoliosis. In the Tay-lor approach, a variant of the standard
paramedian approach described previously, the needle enters 1 cm medial and 1
cm inferior to the posterior supe-rior iliac spine and is directed cephalad and
toward the midline. Reviewing radiographs of the spine before attempting the
block may be useful. Spinal curvature affects the ultimate level by changing
the contour of the subarachnoid space. Previous spinal surgery can similarly
result in technical difficulties
in placing a block. Correctly identifying the
inter-spinous and interlaminar spaces may be difficult at the levels of
previous laminectomy or spinal fusion. The paramedian approach may be easier,
or a level above the surgical site can be chosen. The block may be incomplete,
or the level may be different than anticipated, due to postsurgical anatomic
changes.
Lumbar CSF volume inversely correlates with
the dermatomal spread of spinal anesthesia. Increased intraabdominal pressure
or conditions that cause engorgement of the epidural veins, thus decreasing CSF
volume, are associated with greater dermatomal spread for a given volume of
injectate. This would include conditions such as pregnancy, ascites, and large
abdominal tumors. In these clini-cal situations, higher levels of anesthesia
are achieved with a given dose of local anesthetic than would oth-erwise be
expected. For spinal anesthesia on a term parturient, some clinicians reduce
the dosage of anes-thetic by one-third compared with a nonpregnant patient,
particularly when the block will be initiated with the patient in the lateral
position. Age-related decreases in CSF volume are likely responsible for the
higher anesthetic levels achieved in the elderly for a given dosage of spinal
anesthetic. Severe kypho-sis or kyphoscoliosis can also be associated with a
decreased volume of CSF and often results in a higher than expected level,
particularly with a hypobaric technique or rapid injection. Tradition states that
transient increases in CSF pressure from coughing or straining increase the
spread of local anesthetic in the CSF, but data supporting this are lacking.
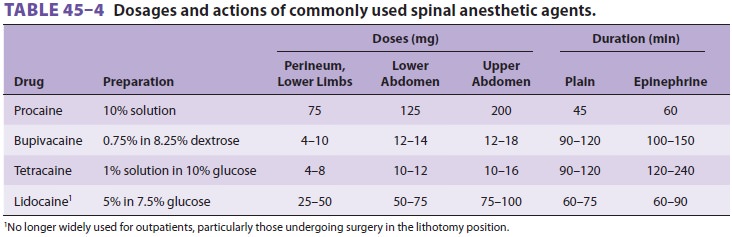
Spinal Anesthetic Agents
Many local anesthetics have been used for
spi-nal anesthesia in the past, but only a few are cur-rently in use (Table
45–4). Only preservative-free local anesthetic
solutions are used. Addition of vasoconstrictors (α-adrenergic agonists, epineph-rine (0.1–0.2 mg)) and opioids enhance the
quality and/or prolong the duration of spinal anesthesia. Vasoconstrictors seem
to delay the uptake of local anesthetics from CSF and may have weak spinal
analgesic properties. Opioids and clonidine can like-wise be added to spinal
anesthetics to improve both the quality and duration of the subarachnoid block.
Hyperbaric bupivacaine and tetracaine are two of the most commonly used
agents for spinal anes-thesia. Both are relatively slow in onset (5–10 min) and
have a prolonged duration (90–120 min). Although both agents produce similar
sensory lev-els, spinal tetracaine more consistently produces motor blockade
than does the equivalent dose of bupivacaine. Addition of epinephrine to spinal
bupivacaine prolongs its duration only modestly. In contrast,
epinephrine can prolong the duration of tetracaine by more than 50%.
Phenylephrine also prolongs tetracaine anesthesia, but has no effect on
bupivacaine spinal blocks. Ropivacaine has also been used for spinal
anesthesia, but experience with it is more limited. Lidocaine and procaine have
a rel-atively rapid onset (3–5 min) and short duration of action (60–90 min).
Their duration is only modestly prolonged by vasoconstrictors. Although
lidocaine spinal anesthesia has been used worldwide, some experts no longer use
this agent because of the phe-nomenon of transient neurological symptoms and
cauda equina syndrome (CES). Repeat lidocainedoses following an initial “failed”
block should be avoided. Indeed,
studies have shown that maldistri-bution of local anesthetic can lead to a
failed spinal in spite of an adequate CSF concentration of local anesthetic.
One alternative agent, 2-chloroprocaine, has been used in some centers with
great success. Unfortunately, older formulations of this agent have produced
cauda equine syndrome when acciden-tally injected intrathecally (in large
doses) during attempted epidural anesthesia.
In North America, hyperbaric spinal anesthesia
is more commonly used than hypobaric or isobaric techniques. The level of
anesthesia is then dependent on the patient’s position during and immediately
fol-lowing the injection. In the sitting position, “saddle block” can be
achieved by keeping the patient sit-ting for 3–5 min following injection, so
that only the lower lumbar nerves and sacral nerves are blocked. If the patient
is moved from a sitting position to a
supine position immediately after injection,
the agent will move more cephalad to the dependent region defined by the
thoracolumbar curve. Hyper-baric anesthetics injected intrathecally with the
patient in a lateral decubitus position are useful for unilateral lower
extremity procedures. The patient is placed laterally, with the extremity to be
operated on in a dependent position. If the patient is kept in this position
for about 5 min following injection, the block will tend to be denser and
achieve a higher level on the operative dependent side.
If regional anesthesia is chosen for surgical pro-cedures involving hip
or lower extremity fracture, hypobaric or isobaric spinal anesthesia can be
use-ful because the patient need not lie on the fractured extremity.
Related Topics