Chapter: Clinical Anesthesiology: Regional Anesthesia & Pain Management: Spinal, Epidural & Caudal Blocks
Clinical Considerations Common to Spinal & Epidural Blocks
Clinical Considerations Common to Spinal & Epidural Blocks
Indications
Neuraxial blocks may be used alone or in
conjunction with general anesthesia for most procedures below the neck. Indeed,
in some centers outside of North America, minimally invasive coronary artery
surgery has been performed with thoracic epidural anesthe-sia alone. As a
primary anesthetic, neuraxial blocks have proved most useful in lower
abdominal, ingui-nal, urogenital, rectal, and lower extremity surgery. Lumbar
spinal surgery may also be performed under spinal anesthesia. Upper abdominal
procedures (eg, gastrectomy) have been performed with spinal or epidural
anesthesia, but because it can be difficult to safely achieve a sensory level
adequate for patient comfort, these techniques are not commonly used.
If a neuraxial anesthetic is being
considered, the risks and benefits must be discussed with the patient, and
informed consent should be obtained. The patient must be mentally prepared for
neuraxial anesthesia, and neuraxial anesthesia must be appro-priate for the
type of surgery. Patients should under-stand that they will have little or no
lower extremity motor function until the block resolves. Proce-dures that
require maneuvers that might compro-mise respiratory function (eg,
pneumoperitoneum or pneumothorax) or are unusually prolonged are typically
performed with general anesthesia, with or without neuraxial blockade.
Contraindications
Major contraindications to neuraxial
anesthe-sia include patient refusal, bleeding diathesis,severe hypovolemia,
elevated intracranial pressure (particularly with an intracranial mass), and
infec-tion at the site of injection. Other relative contrain-dications include
severe aortic or mitral stenosis and severe left ventricular outflow obstruction
(hyper-trophic obstructive cardiomyopathy); however, with close monitoring and
control of the anesthetic level, neuraxial anesthesia can be performed safely
in patients with valvular heart disease, particularly
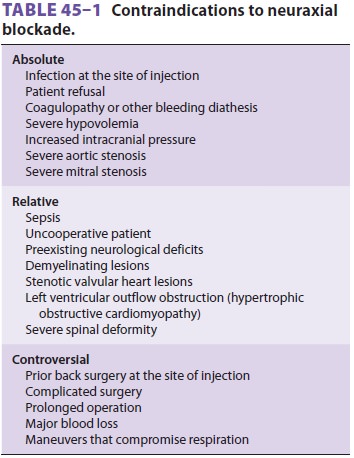
if extensive dermatomal spread of anesthesia is not required (eg,
“saddle” block spinal anesthetics).
Relative and controversial contraindications are also shown in Table
45–1. Inspection and pal-pation of the back can reveal surgical scars,
scolio-sis, skin lesions, and whether the spinous processes can be identified.
Although preoperative screening tests are not required in healthy patients
undergo-ing neuraxial blockade, appropriate testing should be performed if the
clinical history suggests a bleed-ing diathesis. Neuraxial anesthesia in the
presence of sepsis or bacteremia could theoretically predispose patients to
hematogenous spread of the infectious agents into the epidural or subarachnoid
space, as has been shown for lumbar puncture in the presence of septicemia.
Patients with preexisting neurological
deficits or demyelinating diseases may report worsening symptoms following a
block. It may be impossible to discern effects or complications of the block
from preexisting deficits or unrelated exacerbation of pre-existing disease.
For these reasons, some risk-averse practitioners argue against neuraxial
anesthesia in such patients. A preoperative neurological exami-nation should
thoroughly document any deficits. In a retrospective study examining the
records of 567 patients with preexisting neuropathies, 2 of the patients
developed new or worsening neuropathy following neuraxial anesthesia. Although
this find-ing indicates a relatively low risk of further injury, study
investigators suggest that an injured nerve is vulnerable to additional injury,
increasing the likeli-hood of poor neurological outcomes.
Regional anesthesia requires at least some degree of patient
cooperation. This may be difficult or impossible for patients with dementia,
psycho-sis, or emotional instability. The decision must be individualized.
Unsedated young children may not be suitable for pure regional techniques;
however, regional anesthesia is frequently used with general anesthesia in
children.
Neuraxial Blockade in the Setting of Anticoagulants & Antiplatelet Agents
Whether a block should be performed in the set-ting of anticoagulants
and antiplatelet agents can be problematic. The American Society of Regional
Anesthesia and Pain Medicine (ASRA) has issued several guidelines on this
subject. Because guidelines are frequently revised and updated, practitioners
are advised to seek the most recent edition. Although the incidence of epidural
hematoma is reported to be quite low (1 in 150,000 epidurals), ASRA is
concerned that the actual incidence may be somewhat higher. Moreover, the use
of anticoagulant and antiplatelet medications continues to increase, placing an
ever larger number of patients at potential risk of epidural hematomas. Because
of the rarity of epidural hemato-mas, most guidelines are based on expert
opinion and case series reviews, as clinical trials are not feasible.
A. Oral Anticoagulants
If neuraxial anesthesia is to be used in patients receiving warfarin
therapy, a normal prothrombin time and international normalized ratio should be
documented prior to the block. Anesthesia staff should always consult with the
patient’s primary physicians whenever considering the discontinua-tion of
antiplatelet or antithrombotic therapy.
B. Antiplatelet Drugs
By themselves, aspirin and other nonsteroidal antiin-flammatory drugs
(NSAIDs) drugs do not increase the risk of spinal hematoma from neuraxial
anesthesia procedures or epidural catheter removal. This assumes a normal
patient with a normal coagulation profile who is not receiving other
medications that might affect clotting mechanisms. In contrast, more potent
agents should be stopped, and neuraxial blockade should generally be
administered only after their effects have worn off. The waiting period depends
on the specific agent: for ticlopidine (Ticlid), it is 14 days; clopido-grel
(Plavix), 7 days; abciximab (Rheopro), 48 hr; and eptifibatide (Integrilin), 8
hr. In patients with a recently placed cardiac stent, discontinuation of
antiplatelet therapy can result in stent thrombosis and acute ST-segment
elevation myocardial infarction. Risks versus benefits of a neuraxial technique
should be discussed with the patient and the patient’s primary doctors.
C. Standard (Unfractionated) Heparin
“Minidose” subcutaneous heparin prophylaxis is not a contraindication to
neuraxial anesthesia or epidural catheter removal. In patients who are to
receive systemic heparin intraoperatively, blocks may be performed 1 hr or more
before heparin administration. A bloody epidural or spinal does not necessarily
require cancellation of surgery, but discussion of the risks with the surgeon
and careful postoperative monitoring is needed. Removal of an epidural catheter
should occur 1 hr prior to, or 4 hr following, subsequent heparin dosing.
Neuraxial anesthesia should be avoided in
patients on therapeutic doses of heparin and with increased partial
thromboplastin time. If the patient is started on heparin after the placement
of an epi-dural catheter, the catheter should be removed only after discontinuation
or interruption of heparin infu-sion and evaluation of the coagulation status.
The risk of spinal hematoma (with or without neuraxial punc-ture) is unclear in
the setting of full anticoagulation for cardiac surgery. Prompt diagnosis and
evacuation of symptomatic epidural hematomas increase the likelihood that
neuronal function will be preserved.
D. Low-Molecular-Weight Heparin (LMWH)
Many cases of spinal hematoma associated with
neuraxial anesthesia followed the introduction of the
“low-molecular weight heparin” (LMWH)
enoxapa-rin (Lovenox) in the United States in 1993. Many of these cases
involved intraoperative or early post-operative LMWH use, and several patients
were receiving concomitant antiplatelet medication. If an unusually bloody
needle or catheter placement occurs, LMWH should be delayed until 24 hr
post-operatively, because this trauma may increase the risk of spinal hematoma.
If postoperative LMWH thromboprophylaxis will be utilized, epidural catheters
should be removed 2 hr prior to the first LMWH dose. If already present, the
catheter should be removed at least 10 hr after a dose of LMWH, and subsequent
dosing should not occur for another 2 hr.
E. Fibrinolytic or Thrombolytic Therapy
Neuraxial anesthesia should not be performed if a patient has received
fibrinolytic or thrombolytic therapy.
Awake or Asleep?
Should lumbar neuraxial anesthesia, when used
in conjunction with general anesthesia, be performed before or after induction
of general anesthesia? This is controversial. The major arguments for having
the patient asleep are that (1) most patients, if given a choice, would prefer
to be asleep, and (2) the pos-sibility of sudden patient movement causing
injury is markedly diminished. The major argument for neuraxial blockade while
the patient is still awake is that the patient can alert the clinician to
paresthe-sias and pain on injection, both of which have been associated with
postoperative neurological deficits. Although many clinicians are comfortable
perform-ing lumbar epidural or spinal puncture in anes-thetized or deeply
sedated adults, there is greater consensus that thoracic and cervical punctures
should, except under unusual circumstances, only be performed in awake
patients. Pediatric neuraxial blocks, particularly caudal and epidural blocks,
are usually performed under general anesthesia.
Technical Considerations
Neuraxial blocks should be performed only in a facil-ity in which all the equipment and drugs needed for intubation, resuscitation, and general anesthesia are immediately available. Regional anesthesia is greatly facilitated by adequate patient premedication.
Nonpharmacologic patient preparation is also
very helpful. The patient should be told what to expect so as to minimize
anxiety. This is particularly important in situations in which premedication is
not used, as is typically the case in obstetric anesthesia. Supple-mental
oxygen via a face mask or nasal cannula may be required to avoid hypoxemia when
sedation is used. Minimum monitoring requirements include blood pressure and
pulse oximetry for labor anal-gesia. Monitoring for blocks rendered in surgical
anesthesia is the same as that in general anesthesia. Epidural steroid
injections for management of pain (when little or no local anesthetic is
injected) do not require continuous monitoring.
Surface Anatomy
Spinous processes are generally palpable and
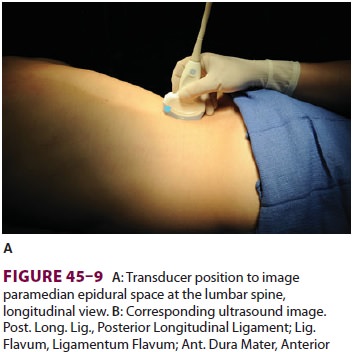
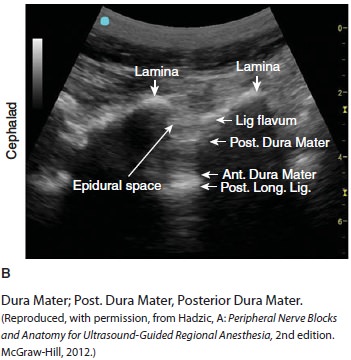
help to define the midline. Ultrasound can be used when landmarks are not
palpable (Figure 45–9). The spi-nous
processes of the cervical and lumbar spine are nearly horizontal, whereas those
in the thoracic spine slant in a caudal direction and can overlap significantly
(Figure 45–2). Therefore, when per-forming a lumbar or cervical epidural block
(with maximum spinal flexion), the needle is directed with only a slight
cephalad angle, whereas for a thoracic block, the needle must be angled
significantly more cephalad to enter the thoracic epidural space. In the
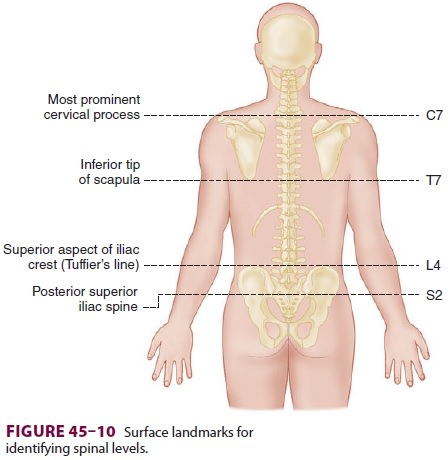
cervical area, the first palpable spinous process is that of C2, but the most
prominent one is that of C7 (vertebra prominens). With the arms at the side,
the spinous process of T7 is usually at the same level as the inferior angle of
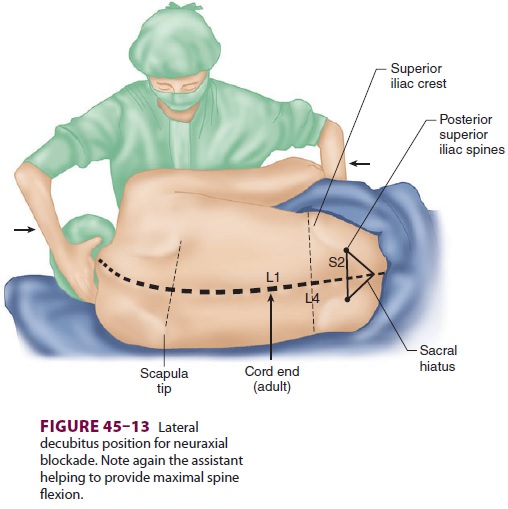
the scapulae ( Figure 45–10). A line drawn between
the highest points of both iliac crests (Tuffier’s line) usually crosses either
the body of L4 or the L4–L5 interspace. Counting spinous processes up or down
from these reference points identifies other spinal levels. A line connecting
the posterior superior iliac spine crosses the S2 posterior foramina. In
slender persons, the sacrum is easily palpable, and the sacral hiatus is felt
as a depression just above or between the gluteal clefts and above the coccyx,
defining the point of entry for caudal blocks.
Patient Positioning
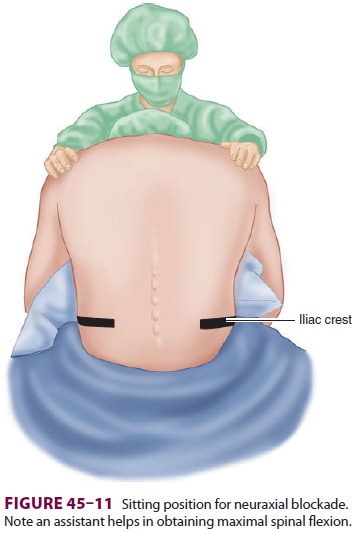
A. Sitting Position
The anatomic midline is often easier to appreciate when the patient is
sitting than when the patient is in the lateral decubitus position ( Figure
45–11). This is particularly true with very obese patients. Patients sit with
their elbows resting on their thighs or a bed-side table, or they can hug a
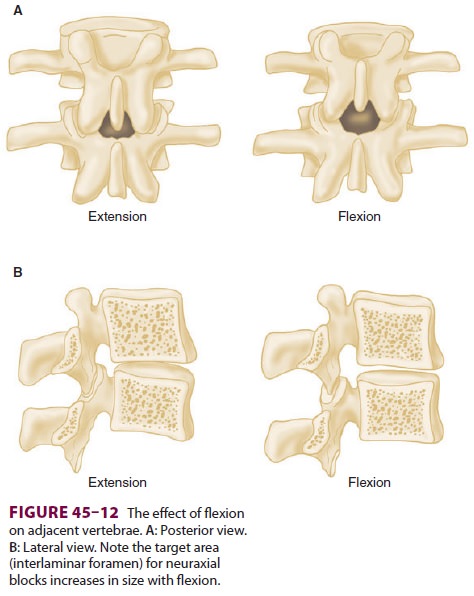
pillow. Flexion of the spine (arching the back “like a mad cat” maximizes the
“target” area between adjacent spinous pro-cesses and brings the spine closer
to the skin surface (Figure 45–12).
B. Lateral Decubitus
Many clinicians prefer the lateral position for neuraxial blocks (Figure
45–13). Patients lie on their side with their knees flexed and pulled high
against the abdo-men or chest, assuming a “fetal position.” An assistant can
help the patient assume and hold this position.
C. Buie’s (Jackknife) Position
This position may be used for anorectal
procedures utilizing an isobaric or hypobaric anesthetic solution . The
advantage is that the block is done in the same position as the operative
procedure, so that the patient does not have to be moved following the block.
The disadvantage is that CSF will not freely flow through the needle, so that
correct subarach-noid needle tip placement will need to be confirmed by CSF aspiration.
A prone position is typically used when fluoroscopic guidance is required.
Anatomic Approach
A. Midline Approach
The spine is palpated, and the patient’s body
position is examined to ensure that the plane of the back is perpendicular to
that of the floor. This ensures that a needle passed parallel to the floor will
stay midline as it courses deeper (Figure 45–4). The depression
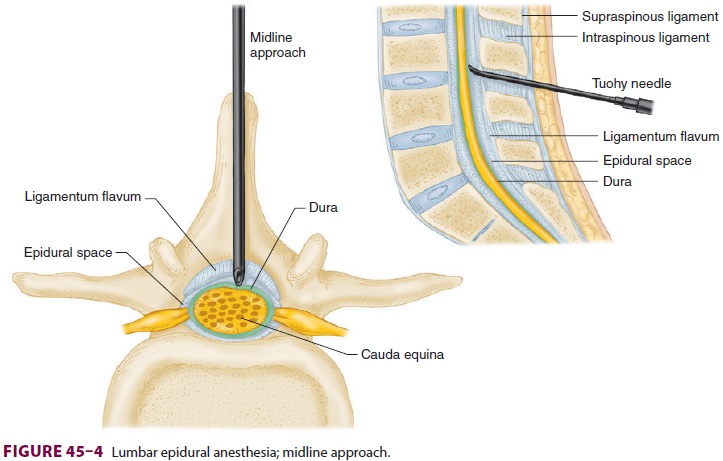
between the spinous processes of the vertebra
above and below the level to be used is palpated; this will be the needle entry
site. A sterile field is established with chlorhexidine or a similar solution.
A fenes-trated sterile drape is applied. After the preparation solution has
dried, a skin wheal is raised at the level of the chosen interspace with local
anesthetic using a small (25-gauge) needle. A longer needle can be used for
deeper local anesthetic infiltration.
Next, the procedure needle is introduced in
the midline. Remembering that the spinous processes course caudad from their
origin at the spine, the needle will be directed slightly cephalad. The
subcu-taneous tissues offer little resistance to the needle. As the needle
courses deeper, it will enter the supraspi-nous and interspinous ligaments,
felt as an increase in tissue resistance. The needle also feels more firmly
implanted in the back. If bone is contacted superfi-cially, a midline needle is
likely hitting the lower spi-nous process. Contact with bone at a deeper level
usually indicates that the needle is in the midline andhitting the upper
spinous process, or that it is lateral to the midline and hitting a lamina. In
either case, the needle must be redirected. As the needle pene-trates the
ligamentum flavum, an obvious increase in resistance is encountered. At this
point, the proce-dures for spinal and epidural anesthesia differ. For epidural
anesthesia, a sudden loss of resis-tance (to injection of air or saline) is
encountered as the needle passes through the ligamentum flavum and enters the
epidural space. For spinal anesthesia, the needle is advanced through the
epi-dural space and penetrates the dura–subarachnoid membranes, as signaled by
freely flowing CSF.
B. Paramedian Approach
The paramedian technique may be selected if
epi-dural or subarachnoid block is difficult, particu-larly in patients who
cannot be positioned easily (eg, severe arthritis, kyphoscoliosis, or prior
spine surgery) (Figure 45–14). Many clinicians
routinely use the paramedian approach for thoracic epidural
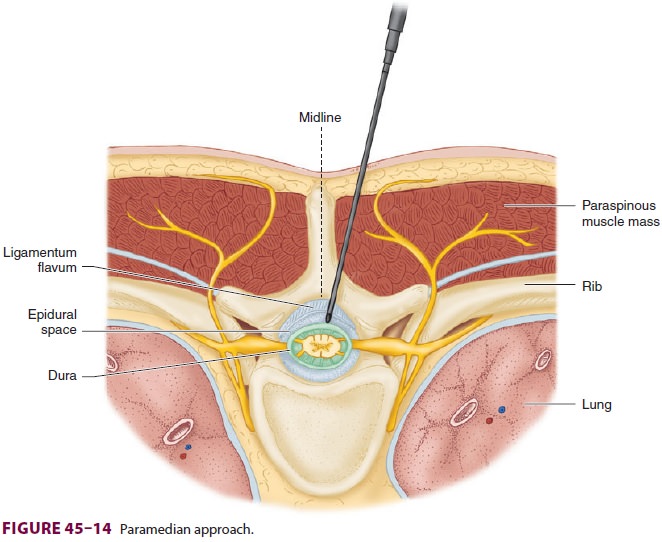
puncture. After skin preparation and sterile
drap-ing (as previously described), the skin wheal for a paramedian approach is
raised 2 cm lateral to the inferior aspect of the superior spinous process of
the desired level. Because this approach is lateral to most of the interspinous
ligaments and pen-etrates the paraspinous muscles, the needle may encounter
little resistance initially and may not seem to be in firm tissue. The needle
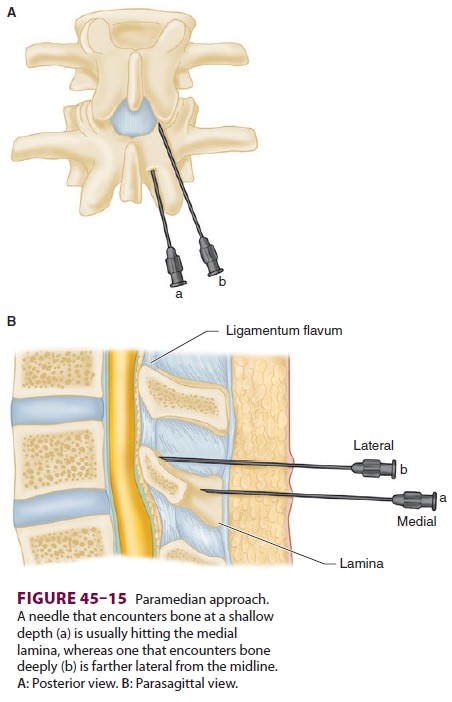
is directed and advanced at a 10–25° angle toward the midline. If bone is
encountered at a shallow depth with the paramedian approach, the needle is
likely in con-tact with the medial part of the lower lamina and should be
redirected mostly upward and perhaps slightly more laterally. On the other
hand, if bone is encountered deeply, the needle is usually in contact with the
lateral part of the lower lamina and should be redirected only slightly
craniad, more toward the midline (Figure 45–15).
C. Assessing Level of Blockade
With knowledge of the sensory dermatomes (see appendix), the extent of
sensory block can be assessed by a blunted needle.
D. Ultrasound-Guided Neuraxial Blockade
Although it has not, as of yet, transformed the prac-tice of neuraxial
blockade in the same manner as it has for other procedures, ultrasound guidance
can facilitate neuraxial blockade in patients with poorly palpable landmarks.
As with other uses of ultra-sound, specific training is required for
practitioners to identify correctly the landmarks and interspaces necessary for
neuraxial blockade.
Related Topics