Chemistry - Solid State: Choose the best answer | 12th Chemistry : UNIT 6 : Solid State
Chapter: 12th Chemistry : UNIT 6 : Solid State
Solid State: Choose the best answer
Chemistry : Solid State
Choose the best answer:
1. Graphite and diamond are
a) Covalent and molecular crystals
b) ionic and covalent crystals
c) both covalent crystals
d) both molecular crystals
2. An ionic compound AxBy crystallizes in fcc type crystal structure with B ions at the centre of each face and A ion occupying entre of the cube. the correct formula of AxBy is
a) AB
b) AB3
c) A3B
d) A8B6
Solution
number of A ions = (Nc/8) = (8/8)=1
number of B ions = (Nf/2) = (6/2) =3
3. The ratio of close packed atoms to tetrahedral hole in cubic packing is
a) 1:1
b) 1:2
c) 2:1
d) 1:4
Solution
if number of close packed atoms =N; then,
The number of Tetrahedral holes formed = 2N
number of Octahedral holes formed = N
therefore N:2N = 1:2
4. Solid CO2 is an example of
a) Covalent solid
b) metallic solid
c) molecular solid
d) ionic solid
Solution
lattice points are occupied by CO2 molecules
5. Assertion : monoclinic sulphur is an example of monoclinic crystal system
Reason: for a monoclinic system, a≠b≠c and α = γ = 90 0 , β ≠ 900
a) Both assertion and reason are true and reason is the correct explanation of assertion.
b) Both assertion and reason are true but reason is not the correct explanation of assertion.
c) Assertion is true but reason is false.
c) Both assertion and reason are false.
6. In calcium fluoride, having the flurite structure the coordination number of Ca2+ ion and F- Ion are
a) 4 and 2
b) 6 and 6
c) 8 and 4
d) 4 and 8
Solution
CaF2 has cubical close packed arrangement
Ca2+ ions are in face centered cubic arrangement, each Ca2+ ions is surrounded by 8 F− ions and each F− ion is surrounded by 4 Ca2+ ions.
Therefore coordination number of Ca2+ is 8 and of F− is 4
7. The number of unit cells in 8 gm of an element X ( atomic mass 40) which crystallizes in bcc pattern is (NA is the Avogadro number)
a) 6.023 X 1023
b) 6.023 X 1022
c) 60.23 X 1023
d) ( [6.023 x 1023 ] / [8 x 40] )
Solution
in bcc unit cell,
2 atoms ≡ 1 unit cell
Number of atoms in 8g of element is ,
Number of moles = 8g / 40 g mol−1 = 0.2 mol
1 mole contains 6.023 ×1023 atoms
0.2 mole contains 0.2 × 6.023 ×1023 atoms
( 1unit cell / 2 atoms ) × 0.2 × 6.023 × 1023
6.023 ×1022 unit cells
8. The number of carbon atoms per unit cell of diamond is
a) 8
b) 6
c) 1
d) 4
Solution
in diamond carbon forming fcc. Carbon occupies corners and face centres and also occupying half of the tetrahedral voids.
(Nc/8) + (Nf/2) + 4 C atomos in Td voids
(8/8) + (6/2) + 4 = 8
9. In a solid atom M occupies ccp lattice and (1/3) of tetrahedral voids are occupied by atom N. find the formula of solid formed by M and N.
a) MN
b) M3N
c) MN3
d) M3N2
Solution
if the total number of M atoms is n, then the number of tetrahedral voids
=2n
given that (1/3)rd of tetrahedral voids are occupied i.e., (1/3)x2n are occupied i.e., by N atoms
∴ M:N ⇒ n : (2/3) n
1 : (2/3)
3 : 2 ⇒ M3 N2
10. The composition of a sample of wurtzite is Fe0.93 O1.00 what % of Iron present in the form of Fe3+?
a) 16.05%
b) 15.05%
c) 18.05%
d) 17.05%
Solution
let
the number of Fe2+ ions in the crystal be x
the number of Fe3+ ions in the crystal be y
total number of Fe2+ and Fe3+ ions is
x + y
given that x + y = 0.93
the total charge =0
x (2+) + (0.93 –x) (+ 3) −2 = 0
2x + 2.97 −3x −2 = 0
x = 0.79
Percentage of Fe3+
= [ ( 0.93 −0.79) / (0.93) ] 100 = 15.05%
11. The ionic radii of A+ and B− are 0.98 × 10−10 m and 1.81 × 10−10 m . the coordination number of each ion in AB is
a) 8
b) 2
c) 6
d) 4
Solution
rC+/ rA- = 0.98 ×10−10 / 1.81 ×10−10 = 0.54
it is in the range of 0. 414 - 0.732 , hence the coordination number of each ion is 6
12. CsCl has bcc arrangement, its unit cell edge length is 400pm, its inter atomic distance is
a) 400pm
b) 800pm
c) √3 x100pm
d) (√3 / 2) x 400pm
Solution
√3 a = rCs+ + 2rCl- + rCs+
(√3/2) a = ( rCl- + rCs+)
(√3/2) x 400 = inter ionic distance
13. A solid compound XY has NaCl structure. if the radius of the cation is 100pm , the radius of the anion will be
a) (100/0.414)
b) (0.732/100)
c) 100x0.414
d) (0.414 / 100)
Solution
for an fcc structure rx+ / ry− = 0.414
given that rX+ = 100 pm
r y− = 100 pm / 0.414
14. The vacant space in bcc lattice unit cell is
a) 48%
b) 23%
c) 32%
d) 26%
Solution
packing efficiency = 68%
therefore empty space percentage = (100-68) = 32%
15. The radius of an atom is 300pm, if it crystallizes in a face centered cubic lattice, the length of the edge of the unit cell is
a) 488.5pm
b) 848.5pm
c) 884.5pm
d) 484.5pm
Solution
let edge length = a
√2a = 4r
a = [4 × 300] / √2
a = 600 ×1.414
a = 848.4 pm
16. The fraction of total volume occupied by the atoms in a simple cubic is
a) π / 4√2
b) π / 6
a) π / 4
a) π / 3√2
Solution

17. The yellow colour in NaCl crystal is due to
a) excitation of electrons in F centers
b) reflection of light from Cl- ion on the surface
c) refraction of light from Na+ ion
d) all of the above
18. if ‘a’ stands for the edge length of the cubic system ; sc , bcc, and fcc. Then the ratio of radii of spheres in these systems will be respectively.
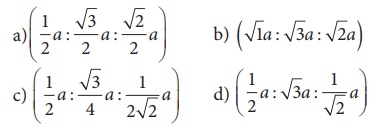
Ans: c) (1/2 a : √3/4 a : 1/2√2 a)
Solution
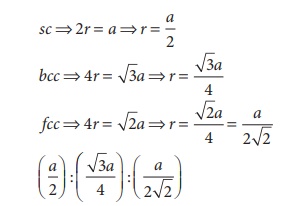
19. If ‘a’ is the length of the side of the cube, the distance between the body centered atom and one corner atom in the cube will be
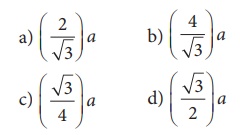
Ans: d) (√3/2)a
Solution
if a is the length of the side, then the length of the leading diagonal passing through the body centered atom is √3a
Required distance (√3/2) a
20. Potassium has a bcc structure with nearest neighbor distance 4.52 Aº . its atomic weight is 39. its density will be
a) 915 kg m-3
b) 2142 kg m-3
c) 452 kg m-3
d) 390 kg m-3
Solution
ρ = n × M / a3NA
for bcc
n = 2
M=39
nearest distance 2r = 4.52
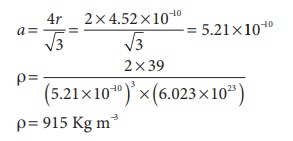
21. Schottky defect in a crystal is observed when
a) unequal number of anions and anions are missing from the lattice
b) equal number of anions and anions are missing from the lattice
c) an ion leaves its normal site and occupies an interstitial site
d) no ion is missing from its lattice.
22. The cation leaves its normal position in the crystal and moves to some interstitial position, the defect in the crystal is known as
a) Schottky defect
b) F center
c) Frenkel defect
d) non-stoichiometric defect
23. Assertion: due to Frenkel defect, density of the crystalline solid decreases.
Reason: in Frenkel defect cation and anion leaves the crystal.
a) Both assertion and reason are true and reason is the correct explanation of assertion.
b) Both assertion and reason are true but reason is not the correct explanation of assertion.
c) Assertion is true but reason is false.
d) Both assertion and reason are false
24. The crystal with a metal deficiency defect is
a) NaCl
b) FeO
c) ZnO
d) KCl
25. A two dimensional solid pattern formed by two different atoms X and Y is shown below. The black and white squares represent atoms X and Y respectively. the simplest formula for the compound based on the unit cell from the pattern is
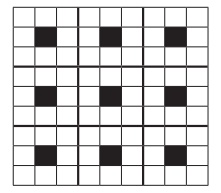
a) XY8
b) X4Y9
c) XY2
d) XY4
PTA
Question Oneword:
1. In FCC unit cell of the edge
length is 8√2 pm. The radius of the metal atom is __________ Ǻ.
a) 0.04
b)
0.02
c)
8 × 10-2
d)
8/√2
Answer: a)
2. The arrangement of
crystallographic axes and angles respectively in hexagonal crystal systems is
a)
a ≠ b ≠ c α = β = γ = 90°
b)
a = b ≠ c α ≠ β ≠ γ = 90°
c) a = b ≠ c α = β = 90° γ = 120°
d)
a = b = c α ≠ β ≠ γ = 90°
Answer: c)
3. The radius of Na+
ion is 95pm and Cl- ion 181 pm. The co-ordination number of Na+
is
a)
4
b) 6
c)
8
d)
3
Answer:
b)
4. The number of close packed
spheres is ‘n'. The number of tetrahedral voids generated is equal to __________
a)
n
b) 2n
c)
2n2
d)
3n
Answer: b)
5. The percentage of packing of
face centred cubic unit cell is ________
a)
52.4%
b)
47.6%
c) 74%
d)
20%
Answer: c)
Related Topics