Chapter: 12th Chemistry : UNIT 6 : Solid State
Classification of solids
Classification
of solids:
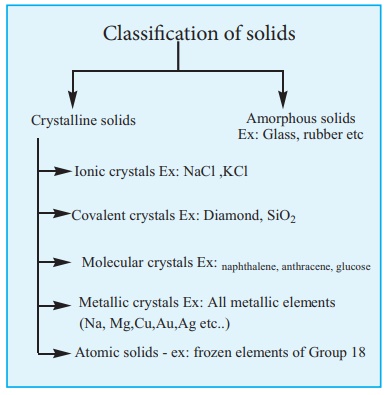
We can classify solids
into the following two major types based on the arrangement of their
constituents.
(i) Crystalline solids
(ii) Amorphous solids.
The term crystal comes
from the Greek word “krystallos” which means clear ice. This term was first
applied to the transparent quartz stones, and then the name is used for solids
bounded by many flat, symmetrically arranged faces.
A crystalline solid is
one in which its constituents (atoms, ions or molecules), have an orderly
arrangement extending over a long range. The arrangement of such constituents
in a crystalline solid is such that the potential energy of the system is at
minimum. In contrast, in amorphous solids (In Greek, amorphous means no form)
the constituents are randomly arranged.
The following table
shows the differences between crystalline and amorphous solids.
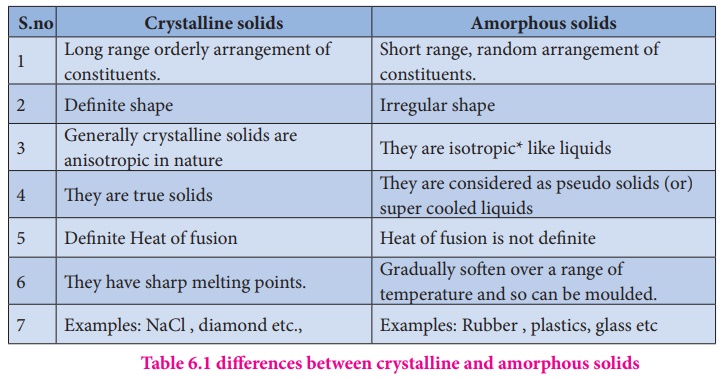
Crystalline solids
1. Long range orderly arrangement of
constituents.
2. Definite shape
3. Generally crystalline solids are
anisotropic in nature
4. They are true solids
5. Definite Heat of fusion
6. They have sharp melting points.
7. Examples: NaCl , diamond etc.,
Amorphous solids
1. Short range, random arrangement
of constituents.
2. Irregular shape
3. They are isotropic* like liquids
4. They are considered as pseudo
solids (or) super cooled liquids
5. Heat of fusion is not definite
6. Gradually soften over a range of
temperature and so can be moulded.
7. Examples: Rubber , plastics,
glass etc
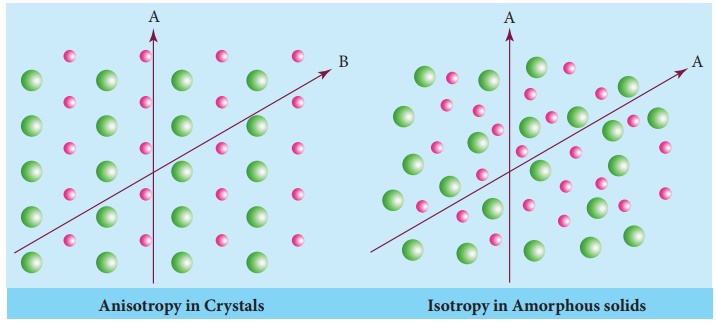
*Isotropy
Isotropy means
uniformity in all directions. In solid state isotropy means having identical
values of physical properties such as refractive index, electrical conductance
etc., in all directions, whereas anisotropy is the property which depends on
the direction of measurement. Crystalline solids are anisotropic and they show
different values of physical properties when measured along different
directions. The following figure illustrates the anisotropy in crystals due to
different arrangement of their constituents along different directions.
Related Topics