Solid State | Chemistry - Imperfection in solids | 12th Chemistry : UNIT 6 : Solid State
Chapter: 12th Chemistry : UNIT 6 : Solid State
Imperfection in solids
Imperfection
in solids:
According to the law of
nature nothing is perfect, and so crystals need not be perfect. They always
found to have some defects in the arrangement of their constituent particles.
These defects affect the physical and chemical properties of the solid and also
play an important role in various processes. For example, a process called
doping leads to a crystal imperfection and it increases the electrical
conductivity of a semiconductor material such as silicon. The ability of
ferromagnetic material such as iron, nickel etc., to be magnetized and
demagnetized depends on the presence of imperfections. Crystal defects are
classified as follows
1) Point defects
2) Line defects
3) Interstitial defects
4) Volume defects
In this portion, we
concentrate on point defects, more specifically in ionic solids. Point defects
are further classified as follows
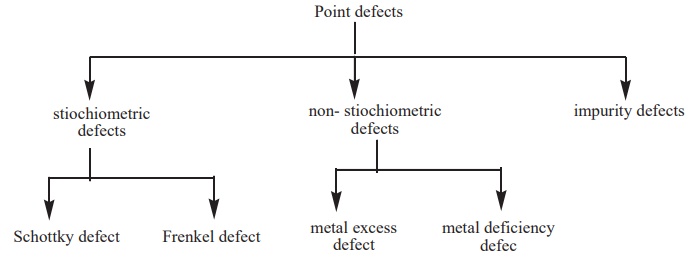
Stoichiometric defects in ionic solid:
This defect is also
called intrinsic (or) thermodynamic defect. In stoichiometric ionic crystals, a
vacancy of one ion must always be associated with either by the absence of
another oppositely charged ion (or) the presence of same charged ion in the
interstitial position so as to maintain the electrical neutrality.
1. Schottky defect:
Schottky defect arises
due to the missing of equal number of cations and anions from the crystal
lattice. This effect does not change the stoichiometry of the crystal. Ionic
solids in which the cation and anion are of almost of similar size show
schottky defect. Example: NaCl.
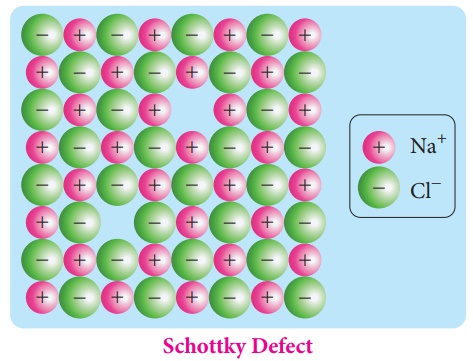
Presence of large number of schottky defects in a crystal, lowers its density. For example, the theoretical density of vanadium monoxide (VO) calculated using the edge length of the unit cell is 6.5 g cm-3, but the actual experimental density is 5.6 g cm-3. It indicates that there is approximately 14% Schottky defect in VO crystal. Presence of Schottky defect in the crystal provides a simple way by which atoms or ions can move within the crystal lattice.
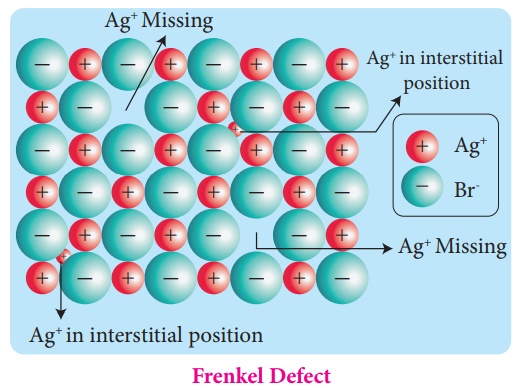
3. Frenkel defect:
Frenkel defect arises
due to the dislocation of ions from its crystal lattice. The ion which is
missing from the lattice point occupies an interstitial position. This defect
is shown by ionic solids in which cation and anion differ in size. Unlike
Schottky defect, this defect does not affect the density of the crystal. For
example AgBr, in this case, small Ag+ ion leaves its normal site and occupies
an interstitial position as shown in the figure.
4. Metal excess defect:
Metal excess defect
arises due to the presence of more number of metal ions as compared to anions.
Alkali metal halides NaCl, KCl show this type of defect.
The electrical
neutrality of the crystal can be maintained by the presence of anionic
vacancies equal to the excess metal ions (or) by the presence of extra cation
and electron present in interstitial position.
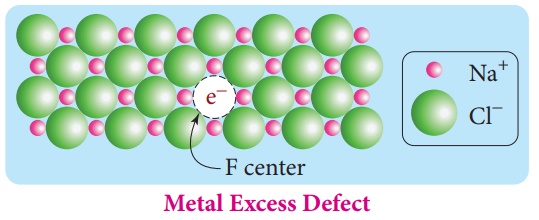
For example, when NaCl
crystals are heated in the presence of sodium vapour, Na+ ions are
formed and are deposited on the surface of the crystal. Chloride ions (Cl-)
diffuse to the surface from the lattice point and combines with Na+ ion. The
electron lost by the sodium vapour diffuse into the crystal lattice and
occupies the vacancy created by the Cl- ions. Such anionic vacancies
which are occupied by unpaired electrons are called F centers. Hence, the
formula of NaCl which contains excess Na+ ions can be written as Na1+ xCl .
ZnO is colourless at
room temperature. When it is heated, it becomes yellow in colour. On heating,
it loses oxygen and thereby forming free Zn2+ ions. The excess Zn2+ ions move
to interstitial sites and the electrons also occupy the interstitial positions.
4. Metal deficiency defect:
Metal deficiency defect
arises due to the presence of less number of cations than the anions. This
defect is observed in a crystal in which, the cations have variable oxidation
states.
For example, in FeO
crystal, some of the Fe2+ ions are missing from the crystal lattice.
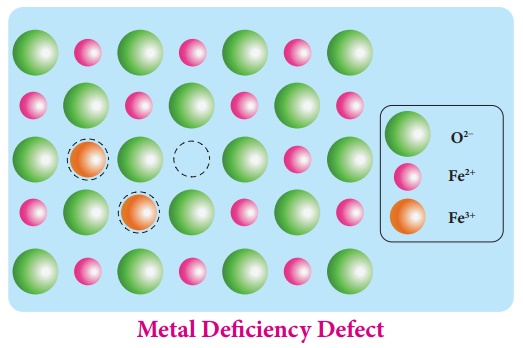
To maintain the electrical
neutrality, twice the number of other Fe2+ ions in the crystal is
oxidized to Fe3+ ions. In such cases, overall number of Fe2+
and Fe3+ ions is less than the O2- ions. It was
experimentally found that the general formula of ferrous oxide is FexO,
where x ranges from 0.93 to 0.98.
5. Impurity defect:
A general method of
introducing defects in ionic solids is by adding impurity ions. If the impurity
ions are in different valance state from that of host, vacancies are created in
the crystal lattice of the host. For example, addition of CdCl2 to
silver chloride yields solid solutions where the divalent cation Cd2+
occupies the position of Ag+. This will disturb the electrical
neutrality of the crystal. In order to maintain the same, proportional number
of Ag+ ions leaves the lattice. This produces a cation vacancy in
the lattice, such kind of crystal defects are called impurity defects.
Related Topics