Chapter: Aquaculture Principles and Practices: Shrimps and Prawns
Reproduction and larval rearing of shrimps
Reproduction and larval rearing of shrimps
The controlled spawning and larval rearing of shrimps was initiated by Hudinaga (1942) with wild spawners of P. japonicus caught from fishing grounds. Since then, as many as 24 Penaeus species and seven Metapenaeus speciesare reported to have been fully or partially propagated artificially. Among these, the more important species for which methods of commercial-scale propagation are available are P. aztecus, P. duorarum, P. indicus, P. japonicusP. kerathurus, P. merguiensis, P. monodon, P. ori-entalis,P. setiferus, P. stylirostris, P. vannamei and M. ensis.
Brood stock
Spawners of P. japonicus, P. aztecus, P. duo-rarum and P. setiferus can be collected in largenumbers, whereas spawners of species like P.monodon are more difficult to obtain. There-fore, maturation of captive stock of wild-caught or pond-reared adults is necessary for large-scale hatchery production of adequate numbers of larvae of these species.
Some species, like P. merguiensis and P. japonicus, mature, mate and spawn freelyin response to controlled environmental conditions. Unilateral eye-stalk ablation is adopted for species which other-wise do not mature in captivity, like P. aztecus,
P. duorarum, P. monodon and P. orientalis. Evenfor species that would mature without such treatment, ablation helps speedy maturation and better spawning rates. By eye-stalk ablation it is possible to reduce the interval between spawnings to 3–15 days, from the normal interval of 10–67 days.
The technique of ablation or extirpation involves the removal of either eye and the partial or total removal of the eye stalk by cutting with surgical scissors, cautery (using a soldering iron or clamps or by electrocautery), ligation, squeezing or crushing the eye stalk tissue, or manual pinching. It is important to prevent excessive loss of eye fluids and infection. The interval between ablation and the onset of maturation and subsequent spawning varies from three days to more than two months, depending on a number of factors including the age of the shrimp and the stage of the moulting cycle. It is considered best to undertake ablation during the intermoult, for
maturation to follow in less than a week. Ablation during the premoult period can lead to immediate moulting and a prolonged latency period. Maturation and viability of eggs seem to depend on the water quality (salinity, temperature and pH), light intensity and nutrition. Spawning stock is given high-quality feed, preferably natural foodstuffs like polychaete worms, squids, mussels, clams or cockle meat, at the rate of about 10 per cent of the biomass. A continuous flow of water is maintained in the maturation tanks and a daily exchange of 60–70 per cent of the water is recommended.
Hatchery systems
Spawning and larval rearing are generally carried out in tanks made of cement concrete, ferrocement, fibreglass, plastic, etc. (fig. 24.8). Maturation cages and pens have been used on an experimental basis, but are

seldom used on a commercial scale. The hatchery tanks used for spawning and larval rearing in Japan are large, ranging from 100 up to 2000 ton capacity. They are suitable for spawning a large number of spawners at a time and for rearing the resultant hatchlings by what is referred to as the ‘community culture method’ (see fig. 6.38). Larval foods are raised by fertilizing the tanks directly every day, producing diatoms and zooplankton, which form the food of larval shrimps. Spawning, larval rearing and fry nursing are all done in the same tank.

Many new hatcheries follow a different system, developed in the USA (Galveston, Texas), in which separate smaller tanks made of fiberglass or plastic are used for spawning and larval rearing. Facilities for culture of live food are maintained separately (fig. 25.9). Larval rearing tanks vary in capacity from 1000 to 2000l and the spawning tanks from 100 to 250l. As high densities of larvae (200–300 nauplii/l) are reared, they cannot be grown beyond the early post-larval stages (for about five days) in the original tanks and further rearing has to be done in nursery tanks or ponds, before grow-out. This type of hatchery system seems to be better suited for species like P. monodon, where the availability of spawnersis very much limited and so community culture may not prove efficient.

Kungvankij (1982) described a third system, which combines the advantages of the above two systems. It includes spawning tanks with capacities of 1000–2000l, larval rearing tanks of 1000–3000l capacity and nursery tanks with a capacity of 30–100 tons for rearing post-larvae to the P30 stage (fig. 25.10). This system is reported to maximize tank utilization in spawning and larval rearing of species like P.monodon
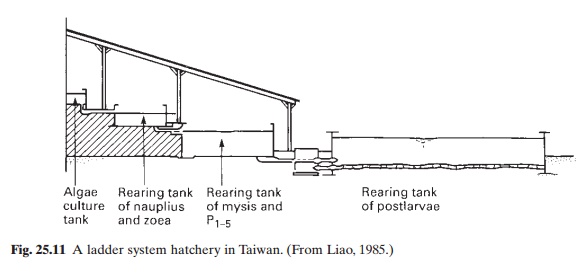
Liao (1985) referred to a recently developed ‘ladder system’ hatchery (fig. 25.11), consisting of four interconnected tanks built on sloping ground, with the algal culture tank at the top, followed below by the rearing tank for nauplii and zoea larvae, then another rearing tank for mysis to the P1–5 stages and finally a larger tank for post-larvae, all built one below the other, with descending water levels.
Spawning and larval rearing
The minimum age of spawning females varies between species and according to the environmental conditions, as can be seen from the following:
In general, spawners from captive brood stock are smaller than those from the wild. Farmers usually believe that spawners from wild stock are superior to captive ablated spawners, and that the quality and quantity of their eggs are higher.
The maturity of the males can be determined by examination of the petasma on the first pair of pleopods. In mature males these accessory organs are joined together by means of inter-locking hooks. Swelling and whitish coloration of the terminal ampoules near the fifth pair of pereiopods indicate gonadal maturity.
The spawning season in nature varies according to species and location. When larvae have to be collected from the wild, or when wild spawners are used for spawning and larval rearing, it is essential to know beforehand the period and locations of their occurrence. On the other hand, captive stocks can be matured and spawned almost throughout the year under controlled conditions. Though tropical species spawn throughout the year, most Penaeids have peak periods of spawning.
In closed thelycum species (i.e. species with lateral plates that lead to a seminal receptacle, where the spermatophores can be inserted), the mating occurs soon after the females have moulted. In species with an open thelycum (with only ridges and protuberances for spermatophore attachment) mating can occur soon
after the eggs become mature. In the latter group of species, spermatophores can easily be lost or fail to be affixed before spawning. The spermatophores deposited during a single moulting are generally enough, irrespective of the moult cycle, to fertilize up to three successive spawns.
For controlled spawning, gravid females and males in advanced stages of maturity are stocked in spawning tanks. Spawners obtained from commercial catches during the winter are likely to be infected and are therefore usually treated with 3ppm KMnO4, 25ppm formalin or the commercial product Treflan®(trifuralin) at concentrations of 3–5ppm. In large tank systems used for community culture, several spawners are introduced into the community tank, whereas in the other systems individual spawners or batches of spawners are placed in separate spawning tanks each time. In large tanks, the density of spawners are generally:
P. japonicus: 1 spawner/2m3
P. monodon: 1 spawner/5m3
P. indicus: 1 spawner/1m3
P. merguiensis: 1 spawner/1m3
Generally a 1:1 sex ratio is maintained in spawning tanks, but a ratio of two females to one male has produced higher spawning rates and egg production. There is usually a time lag between mating and spawning, as the eggs may still not be fully mature at the time of mating.
Penaeus japonicus and P. indicus females havebeen observed to eat their own spawned eggs and so it is advisable to install mesh trays or plates on the bottom of the spawning tanks to protect the eggs. The salinity in the tanks gen-erally ranges from 28 to 35ppt and the temperature from 23 to 33°C. Spawning usually takes place at night. Fertilization is external and at the above temperature range the embryonic development is rapid.
The nauplius passes through three to six sub-stages (N1–N6) and subsists on its own yolk material. In about two to three days it metamorphoses into protozoea with three substages (PZ1–PZ3) during which period the larva starts feeding on unicellular algae. This stage, which lasts for three to six days is succeeded by the mysis stage with three sub-stages (M1–M3). During this stage the larva retains the filteringmechanism for feeding on algal cells. The mysis metamorphoses into post-larva in about three to five days. At this stage it ceases to be a filter-feeder and becomes capable of capturing and eating zooplankton. Development from the post-larval stage to the juvenile stage is very gradual and a PL5 (P1–P5 or PL1–PL5 denotes the post-larval age in days) may take 15–20 days to reach a size of 20–25mm, suitable for stocking production ponds.
In larval culture of most Penaeids, the main difficulty is in rearing the protozoeal stage when they start feeding. At this stage the larva is highly light-sensitive, and so the tanks should be properly covered to ensure darkness. The key to the success of the pioneer experiments of Hudinaga in Japan was the method developed for the culture of the diatom Skeletonemacostatum, which formed a suitable food for thelarvae. Since then several other types of live foods and feedstuffs have been tried, but cultured phytoplankton appears to be still the most efficient food for larvae at this stage. Since the size of larvae of different species of Penaeids are not the same, they require phyto-plankton of different sizes. They start feeding on zooplankton when they reach the last sub-stage of protozoea. Both mysis and post-larvae up to the fifth day prefer zooplankton, but after that stage they will consume larger food and may feed at the bottom. They can then be fed on polychaetes, chopped mussels, clams, cockles and artificial compound diets.
The more important phytoplankters suitable as food for shrimp larvae are species of Chaetoceros, Skeletonema and Tetraselmis.Algal culture methods have been described. Among the zooplanktonic organisms, the rotifer Brachionus plicatilis is probably the most important as larval food. Many hatcheries depend largely on the brine shrimp,Artemia salina, the nauplii of which form excellent food for shrimp larvae. Methods of culturing Brachionus and hatching Artemia cysts have been described. In large tank hatcheries practising community culture, the tanks are fertilized soon after hatching, at the rate of 3ppm KNO3 and 0.3ppm Na2HPO4 to produce the phytoplankton needed to feed the larvae when they reach the protozoea stage. In some hatcheries pure cultures of diatoms are inoculated before fertilizers are applied. The
production of phytoplankton is maintained through additional fertilization if needed. If the density of plankton is inadequate, supplementary feed in the form of soybean cake, soybean curd, egg yolk of fertilized eggs of oysters may be given.When the larvae reach the mysis stage, they are fed on Brachionus or brine shrimp nauplii. In most hatcheries, post-larval stages are fed on brine shrimp and after the P6 stage on minced mussels, clam meat or formulated larval feeds, partly replacing brine shrimp nauplii until they reach the P7 stage. Beyond this stage, the post-larvae are fed only minced mussel, clam meat or artificial diets, three or four times daily.
As indicated earlier, in hatcheries with separate small hatchery tanks, algae (Skeletonemacostatum and Tetraselmis spp.) are cultured inseparate algal tanks or in plastic bags and the required quantities are introduced daily during the protozoea stage. Artemia nauplii hatched in special tanks are fed to mysis and early post-larval stages. In hatcheries with intermediate size tanks, fertilization of the tank water and introduction of pure algal cultures are combined, and the post-larvae are reared up to the P25stage.
Hatcheries that produce only P5 or P6 stage post-larvae use concrete tanks, earthen ponds or net cages for larval rearing. Small tanks with a filtered sea-water supply and aeration are stocked at a density of up to 150/l. Diatom cultures are introduced to feed the larvae and often a substrate such as polyethylene netting is provided for the larvae to rest on. Early post-larvae are fed with chopped mussel and cockle meat together with young and adult Artemia. Daily exchange of water is maintained for the duration of culture (about 30 days).
Earthen nursery ponds range in area from 500 to 2000m2 with an average depth of 40– 70cm. The larvae are stocked at the P9–P10 stage at densities of 100–150 per m2. The ponds are prepared by eradicating predators and fertilizing with a combination of organic manures (such as 1000kg/ha of chicken manure) and inorganic fertilizer (such as 50kg/ha of ammonium sulphate). Supplementary feeding is done with chopped mussel or cockle meat, at about 10 per cent of the total biomass. The larvae can be reared in such ponds up to the P40 or P60 stages.
Twenty-one- to twenty-five-day-old post-larvae are suitable for stocking grow-out ponds. In some farms, particularly in Ecuador and Taiwan, post-larvae are grown (sometimes referred to as pre-growing) for 30–60 days at densities of 50–200 per m2. With daily exchange of water (10–40 per cent) and supplementary feeding with compound feeds, they reach a mean weight of 0.5–2g, with a survival of 80 per cent depending on species and pond conditions. These fry are then stocked in grow-out facilities.
Nursery cages are used only rarely, as the very small mesh sizes required can become rapidly choked by bio-fouling. The cages, when used, are rectangular in shape (1–2m x 5m x 1m) and are of the floating or stationary type installed in protected bays, lagoons or ponds. Post larvae (P6–P7) are stocked at higher densities of 1000–2000 per m3. Feeding is carried out in the same manner as in earthen ponds.
Related Topics