Structure, Denaturation, Importance - Proteins | 12th Chemistry : UNIT 14 : Biomolecules
Chapter: 12th Chemistry : UNIT 14 : Biomolecules
Proteins
Proteins
Proteins are most abundant biomolecules in all living organisms. The
term protein is derived from Greek word ‘Proteious’
meaning primary or holding first place. They are main functional units for the
living things. They are involved in every function of the cell including
respiration. Proteins are polymers of α-amino acids.
Amino acids
Amino acids are compounds which contain an amino group and a carboxylic
acid group. The protein molecules are made up α-amino acids which can be represented by the
following general formula.
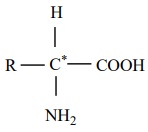
There are 20 α-amino acids commonly found in the protein molecules. Each amino acid is given a trivial name, a three letter code and a one letter code. In writing the amino acid sequence of a protein, generally either one letter or three letter codes are used.
Classification of proteins
Proteins are classified based on their structure (overall shape) into
two major types. They are fibrous proteins and globular proteins.
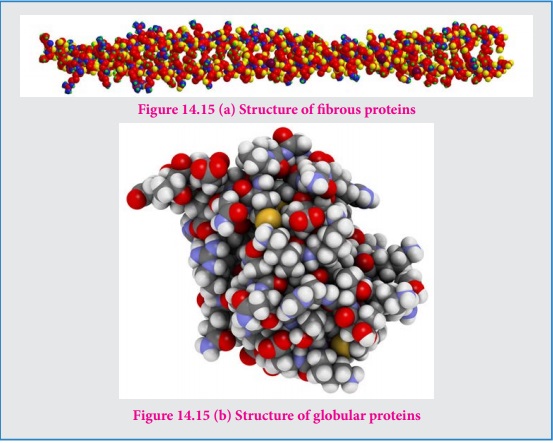
Fibrous proteins
Fibrous proteins are linear molecules similar to fibres. These are
generally insoluble in water and are held together by disulphide bridges and
weak intermolecular hydrogen bonds. The proteins are often used as structural
proteins. Example: Keratin, Collagen etc…
Globular proteins
Globular proteins have an overall spherical shape. The polypeptide chain
is folded into a spherical shape. These proteins are usually soluble in water
and have many functions including catalysis (enzyme). Example: myoglobin,
insuline
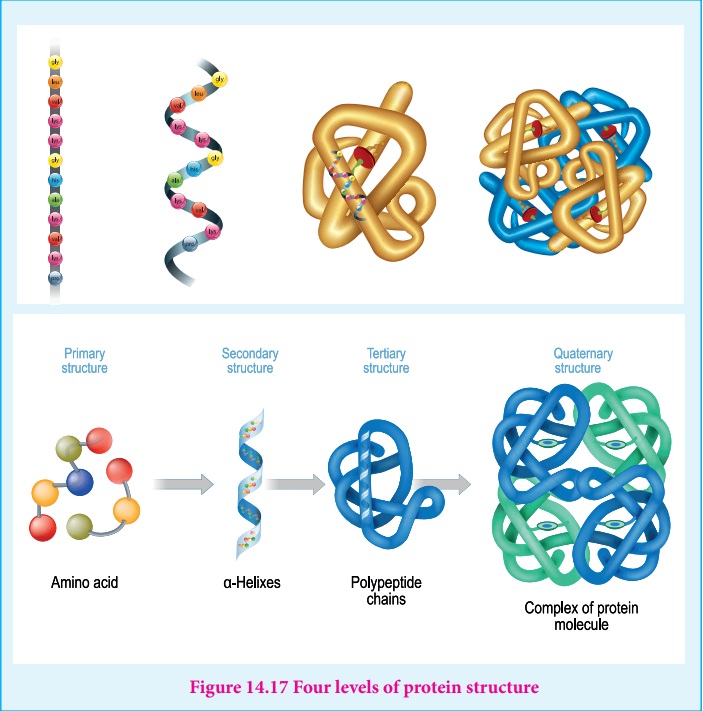
Structure of proteins
Proteins are polymers of amino acids. Their three dimensional structure
depends mainly on the sequence of amino acids (residues). The protein structure
can be described at four hierarchal levels called primary, secondary, tertiary
and quaternary structures as shown in the figure 14.16
1. Primary structure of proteins:
Proteins are polypeptide chains, made up of amino acids are connected
through peptide bonds. The relative arrangement of the amino acids in the
polypeptide chain is called the primary structure of the protein. Knowledge of
this is essential as even small changes have potential to alter the overall
structure and function of a protein.
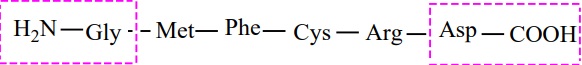
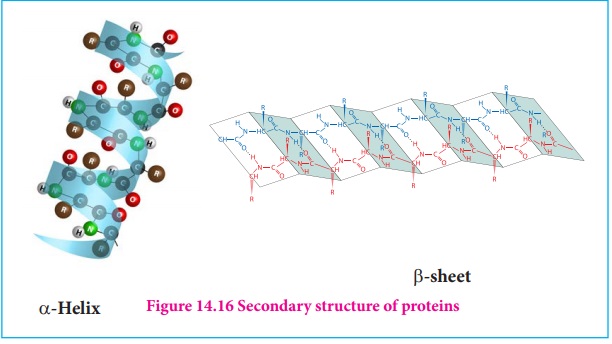
2. Secondary structure of proteins:
The amino acids in the polypeptide chain forms highly regular shapes
(sub-structures) through the hydrogen bond between the carbonyl oxygen
(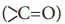 ) and the neighbouring amine hydrogen (-NH)of the main chain.α-Helix
and β-strands
or sheets are two most common sub-structures formed by proteins.
) and the neighbouring amine hydrogen (-NH)of the main chain.α-Helix
and β-strands
or sheets are two most common sub-structures formed by proteins.
α-Helix
In the α-helix
sub-structure, the amino acids are arranged in a right handed helical (spiral)
structure and are stabilised by the hydrogen bond between the carbonyl oxygen
of one amino acid (nth residue) with amino hydrogen of the fifth
residue (n+4th residue). The side chains of the residues protrude
outside of the helix. Each turn of an α-helix contains about 3.6 residues and is about 5.4
Aº long. The amino acid proline produces a kink in
the helical structure and often called as a helix breaker due to its rigid
cyclic structure.
β-Strand
β-Strands
are extended peptide chain rather than coiled. The hydrogen bonds occur between
main chain carbonyl group one such strand and the amino group of the adjacent
strand resulting in the formation of a sheet like structure. This arrangement
is called β-sheets.
3. Tertiary structure:
The secondary structure elements (α-helix & β-sheets) further folds to form the three
dimensional arrangement. This structure is called tertiary structure of the
polypeptide (protein). Tertiary structure of proteins are stabilised by the
interactions between the side chains of the amino acids. These interactions
include the disulphide bridges between cysteine residues, electrostatic,
hydrophobic, hydrogen bonds and van der Waals interactions.
4. Quaternary Structure
Some proteins are made up of more than one polypeptide chains. For
example, the oxygen transporting protein, haemoglobin contains four polypeptide
chains while DNA polymerase enzyme that make copies of DNA, has ten polypeptide
chains. In these proteins the individual polypeptide chains (subunits)
interacts with each other to form the multimeric structure which are known as
quaternary structure. The interactions that stabilises the tertiary structures
also stabilises the quaternary structures.
Denaturation of proteins
Each protein has a unique three-dimensional structure formed by
interactions such as disulphide bond, hydrogen bond, hydrophobic and
electrostatic interactions. These interactions can be disturbed when the
protein is exposed to a higher temperature, by adding certain chemicals such as
urea, alteration of pH and ionic strength etc., It leads to the loss of the
three-dimensional structure partially or completely. The process of a losing
its higher order structure without losing the primary structure, is called
denaturation. When a protein denatures, its biological function is also lost.
Since the primary structure is intact, this process can be reversed in
certain proteins. This can happen spontaneously upon restoring the original
conditions or with the help of special enzymes called cheperons (proteins that
help proteins to fold correctly).
Example: coagulation of egg white by action of heat.

Importance of proteins
Proteins are the functional units of living things and play vital role
in all biological processes
• All biochemical reactions occur in the living systems
are catalysed by the catalytic proteins called enzymes.
• Proteins such as keratin, collagen act as
structural back bones.
• Proteins are used for transporting molecules
(Haemoglobin), organelles (Kinesins) in the cell and control the movement of molecules
in and out of the cells (Transporters).
• Antibodies help the body to fight various
diseases.
• Proteins are used as messengers to coordinate
many functions. Insulin and glucagon control the glucose level in the blood.
• Proteins act as receptors that detect presence of
certain signal molecules and activate the proper response.
• Proteins are also used to store metals such as iron (Ferritin) etc.
Related Topics