Preparation, Structure, Cyclic structure of glucose - Glucose | 12th Chemistry : UNIT 14 : Biomolecules
Chapter: 12th Chemistry : UNIT 14 : Biomolecules
Glucose
Glucose
Glucose is a simple sugar which serves as a major energy source for us.
It is the most important and most abundant sugar. It is present in honey, sweet
fruits such as grapes and mangoes etc... Human blood contains about 100 mg/ dL
of glucose, hence it is also known as blood sugar. In the combined form it is
present in sucrose, starch, cellulose etc.,
Preparation of glucose
1. When sucrose (cane sugar)
is boiled with dilute H 2 SO4 in alcoholic solution, it
undergoes hydrolysis and give glucose and fructose.
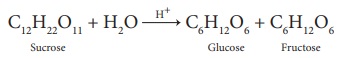
2. Glucose is produced commercially by the hydrolysis of starch with
dilute HCl at high temperature under pressure.
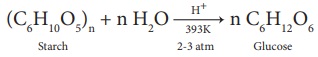
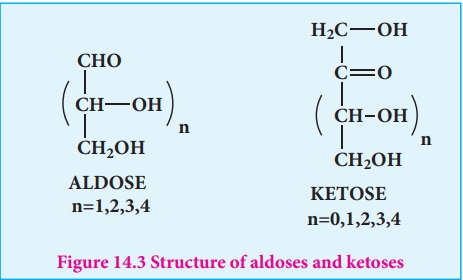
Structure of Glucose
Glucose is an aldohexose. It is optically active with four asymmetric
carbons. Its solution is dextrorotatory and hence it is also called as dextrose.
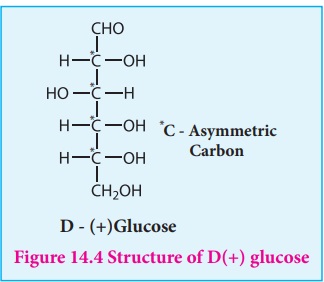
The proposed structure of glucose is shown in the figure 14.4 which was derived
based on the following evidences.
1. Elemental analysis and molecular weight determination show that the
molecular formula of glucose is C 6 H12 O6 .
2. On reduction with concentrated HI and red phosphorus at 373K, glucose
gives a mixture of n hexane and 2–iodohexane indicating that the six carbon
atoms are bonded linearly.
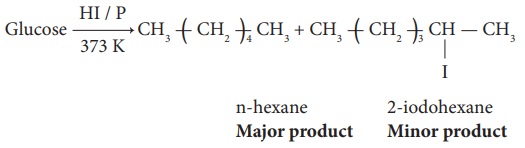
3. Glucose reacts with hydroxylamine to form oxime and with HCN to form
cyanohydrin. These reactions indicate the presence of carbonyl group in
glucose.
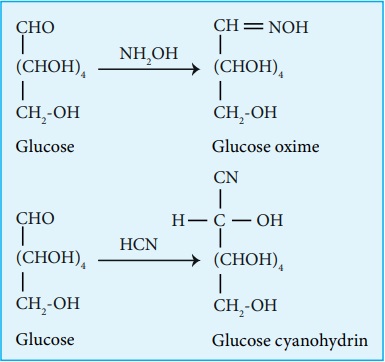
4. Glucose gets oxidized to gluconic acid with mild oxidizing agents
like bromine water suggesting that the carbonyl group is an aldehyde group and
it occupies one end of the carbon chain. When oxidised using strong oxidising
agent such as conc. nitric acid gives glucaric acid (saccharic acid) suggesting
the other end is occupied by a primary alcohol group.
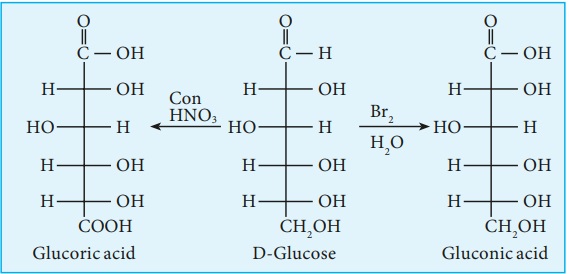
5. Glucose is oxidised to gluconic acid with ammonical silver nitrate
(Tollen’s reagent) and alkaline copper sulphate (Fehling’s solution). Tollen’s
reagent is reduced to metallic silver and Fehling’s solution to cuprous oxide
which appears as red precipitate. These reactions further confirm the presence
of an aldehyde group
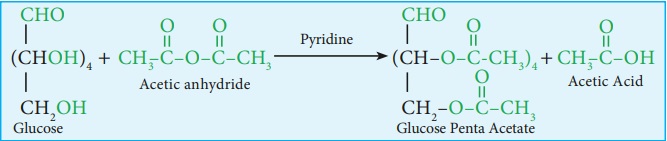
6. Glucose forms penta acetate with acetic anhydride suggesting the
presence of five alcohol groups.
7. Glucose is a stable compound and does not
undergo dehydration easily. It indicates that not more than one hydroxyl group
is bonded to a single carbon atom. Thus the five the hydroxyl groups are
attached to five different carbon atoms and the sixth carbon is an aldehyde
group.
8. The exact spacial arrangement of -OH groups was given by Emil Fischer
as shown in Figure 14.4. The glucose is referred to as D(+) glucose as it has D
configuration and is dextrorotatory.
Cyclic structure of glucose
Fischer identified that the open chain penta hydroxyl aldehyde structure
of glucose, that he proposed, did not completely explain its chemical
behaviour. Unlike simple aldehydes, glucose did not form crystalline bisulphite
compound with sodium bisulphite. Glucose does not give Schiff’s test and the
penta acetate derivative of glucose was not oxidized by Tollen’s reagent or
Fehling’s solution. This behaviour could not be explained by the open chain
structure.
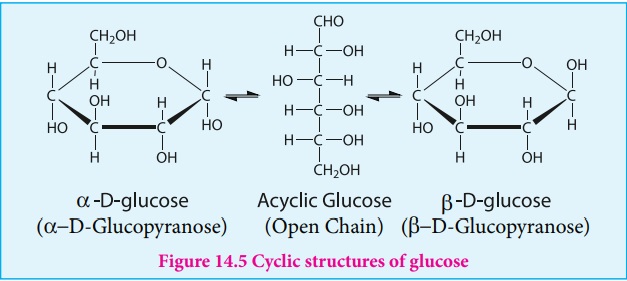
In addition, glucose is found to crystallise in two different forms
depending upon the crystallisation conditions with different melting points
(419 and 423 K). In order to explain these it was proposed that one of the
hydroxyl group reacts with the aldehyde group to form a cyclic structure
(hemiacetal form) as shown in figure 14.5. This also results in the conversion
of the achiral aldehyde carbon into a chiral one leading to the possibility of
two isomers. These two isomers differ only in the configuration of C1 carbon.
These isomers are called anomers. The
two anomeric forms of glucose are called
a and β-forms.
This cyclic structure of glucose is
similar to pyran, a cyclic compound with 5 carbon and one oxygen atom, and
hence is called pyranose form. The specific rotation of pure α- and β-(D)
glucose are 112º and 18.7º respectively. However, when a pure form of any one of
these sugars is dissolved in water, slow interconversion of α-D
glucose and β-D
glucose via open chain form occurs until equilibrium is established giving a
constant specific rotation + 53º. This phenomenon is called mutarotation.
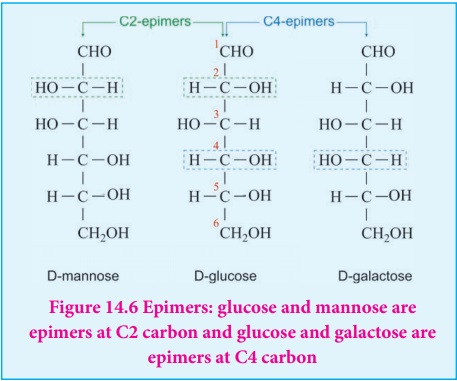
Epimers and epimerisation:
Sugar differing in configuration at an asymmetric centre is known as
epimers. The process by which one epimer is converted into other is called
epimerisation and it requires the enzymes epimerase. Galactose is converted to
glucose by this manner in our body.
Related Topics