Preparation, Structure, Cyclic structure of fructose - Fructose | 12th Chemistry : UNIT 14 : Biomolecules
Chapter: 12th Chemistry : UNIT 14 : Biomolecules
Fructose
Fructose
Fructose is another commonly known monosaccharide having the same
molecular formula as glucose. It is levorotatory and a ketohexose. It is
present abundantly in fruits and hence it is also called as fruit sugar.
Preparation
1. From sucrose
Fructose is obtained from sucrose by heating with dilute H 2
SO4 or with the enzyme invertase
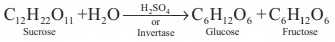
Fructose is separated by crystallisation. The mixture having equal
amount of glucose and fructose is termed as invert sugar.
2. From Inulin
Fructose is prepared commercially by hydrolysis of Inulin (a
polysaccharide) in an acidic medium.
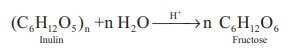
Structure of fructose:
Fructose is the sweetest of all known sugars. It is readily soluble in
water. Fresh solution of fructose has a specific rotation -133º which changes to – 92 º at equilibrium due to
mutarotation. Similar to glucose the structure of fructose is deduced from the
following facts.
1. Elemental analysis and molecular weight
determination of fructose show that it has the molecular formula C 6
H12 O6
2. Fructose on reduction with HI and red phosphorus gives a mixture of n
– hexane (major product) and 2 – iodohexane (minor product). This reaction
indicates that the six carbon atoms in fructose are in a straight chain.
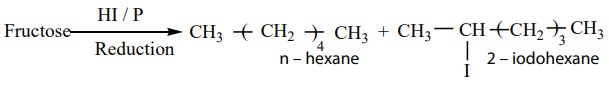
3. Fructose reacts with NH2OH and HCN .
It shows the presence of a carbonyl groups in the fructose.
4. Fructose reacts with acetic anhydride in the
presence of pyridine to form penta acetate. This reaction indicates the
presence of five hydroxyl groups in a fructose molecule.
5. Fructose is not oxidized by bromine water. This
rules out the possibility of absence of an aldehyde (-CHO) group.
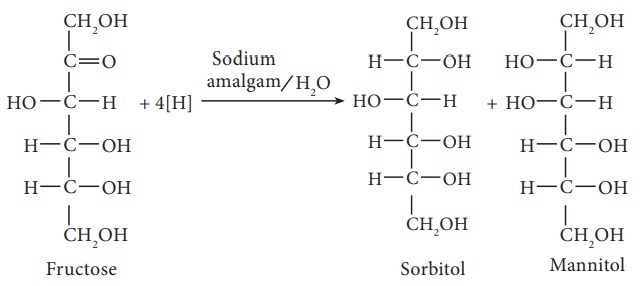
6. Partial reduction of fructose with sodium amalgam and water produces
mixtures of sorbitol and mannitol which are epimers at the second carbon. New
asymmetric carbon is formed at C-2. This confirms the presence of a keto group.
7. On oxidation with nitric acid, it gives glycollic acid and tartaric
acids which contain smaller number of carbon atoms than in fructose.
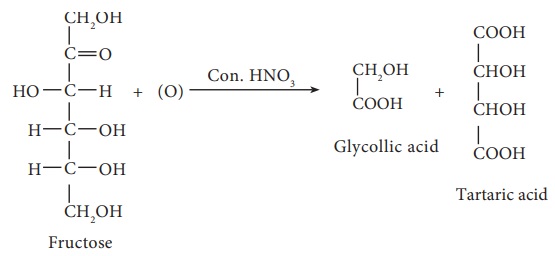
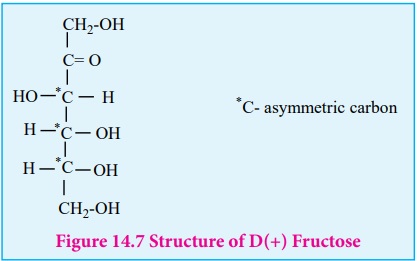
This shows that a keto group is present in C-2. It also shows that 1O
alcoholic groups are present at C- 1 and C- 6. Based on these evidences, the
following structure is proposed for fructose (Figure 14-7)
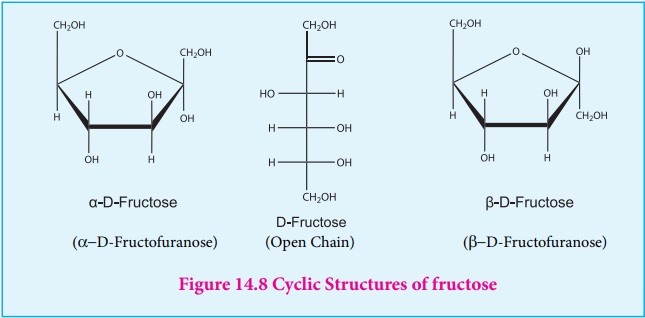
Cyclic structure of fructose
Like glucose, fructose also forms cyclic structure. Unlike glucose it
forms a five membered ring similar to furan. Hence it is called furanose form.
When fructose is a component of a saccharide as in sucrose, it usually occurs
in furanose form.
Related Topics