Structure, Configuration, Classification | Biomolecules | Chemistry - Carbohydrates | 12th Chemistry : UNIT 14 : Biomolecules
Chapter: 12th Chemistry : UNIT 14 : Biomolecules
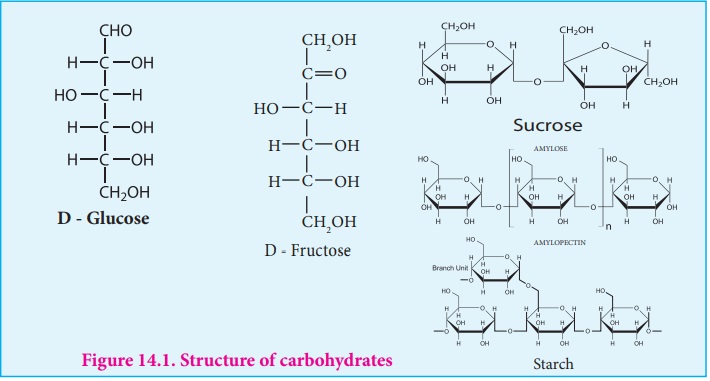
Carbohydrates
Carbohydrates:
Carbohydrates are the most abundant organic compounds in every living
organism. They are also known as saccharides (derived from Greek word ‘ sakcharon’ which means sugar) as many of
them are sweet. They are considered as hydrates of carbon, containing hydrogen
and oxygen in the same ratio as in water. Chemically, they are defined as
polyhydroxy aldehydes or ketones with a general formula Cn(H2O)n.
Some common examples are glucose (monosaccharide), sucrose (disaccharide) and
starch (polysaccharide)
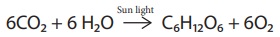
Carbohydrates are synthesised by green leaves during photo synthesis, a
complex process in which sun light provides the energy to convert carbon
dioxide and water into glucose and oxygen. Glucose is then converted into other
carbohydrates and is consumed by animals.
6CO2
+ 6 H2O ------Sun light→ C2H12O6
+ 6O2
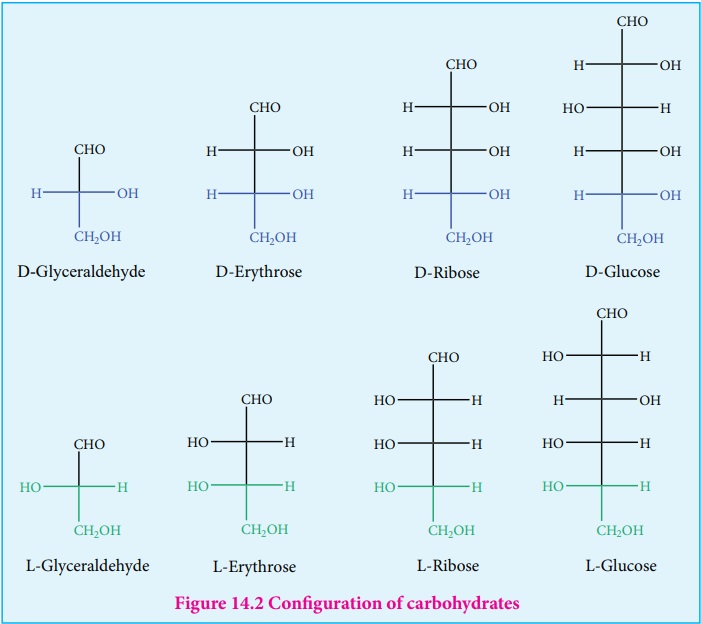
Configuration of carbohydrates:
Almost all carbohydrates are optically active as they have one or more
chiral carbons. The number of optical isomers depends on the number of chiral
carbons (2n isomers, where n is the total number of chiral carbons).
We have already learnt in XI standard to represent an organic compound using
Fischer projection formula. Fischer has devised a projection formula to relate
the structure of a carbohydrate to one of the two enantiomeric forms of glyceraldehyde
(Figure 14.2). Based on these structures, carbohydrates are named as D or L.The
carbohydrates are usually named with two prefixes namely D or L and followed by
sign either (+) or (-). Carbohydrates are assigned the notation (D/L) by comparing
the configuration of the carbon that is attached to -CH2OH group
with that of glyceraldehyde.For example D-glucose is so named because the H and
OH on C5 carbon are in the same configuration as the H and OH on C2 carbon in
D-Glyceraldehyde.
There + and – sign indicates the dextro rotatory and levo rotatory
respectively. Dextro rotatory compounds rotate the plane of plane polarised
light in clockwise direction while the levo rotatory compounds rotate in
anticlockwise direction. The D or L isomers can either be dextro or levo
rotatory compounds. Dextro rotatory compounds are represented as D-(+) or L-(+)
and the levo rotatory compounds as D-(–) or L-(–)
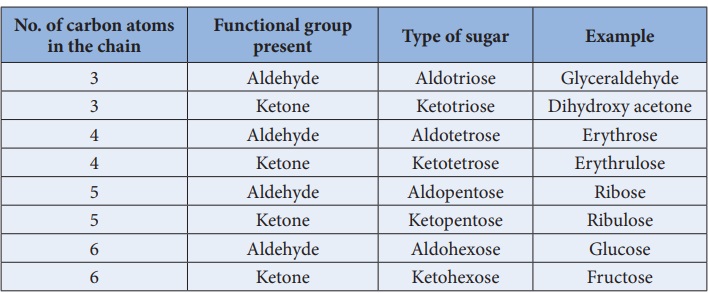
Classification of carbohydrates:
Carbohydrates can be classified into three major groups based on their
product of hydrolysis, namely monosaccharides, oligosaccharides and
polysaccharides.
Monosaccharides:Monosaccharides
are carbohydrates that cannot be hydrolysed further and are also called simple sugars. Monosaccharides have general formula
C n(H2O)n. While there are many
monosaccharides known only about 20 of them occur in nature. Some common
examples are glucose, fructose, ribose, erythrose
Monosaccharides are further classified based on the functional group
present (aldoses or ketoses) and the number of carbon present in the chain
(trioses, tetroses, pentoses, hexoses etc...). If the carbonyl group is an
aldehyde, the sugar is an aldose. If the carbonyl group is a ketone, the sugar
is a ketose. The most common monosaccharides have three to eight carbon atoms.
Table 14.1 Different types of monosaccharides:
Related Topics