Chapter: Essentials of Anatomy and Physiology: Some Basic Chemistry
Proteins
PROTEINS
Proteins are made of smaller subunits or building blocks called amino acids, which all contain the elements carbon, hydrogen, oxygen, and nitrogen. Some amino acids contain sulfur, which permits the forma-tion of disulfide bonds. There are about 20 amino acids that make up human proteins. The structure of amino acids is shown in Fig. 2–8. Each amino acid has a central carbon atom covalently bonded to an atom of hydrogen, an amino group (NH2), and a carboxyl group (COOH). At the fourth bond of the central car-bon is the variable portion of the amino acid, repre-sented by R. The R group may be a single hydrogen atom, or a CH3 group, or a more complex configura-tion of carbon and hydrogen. This gives each of the 20 amino acids a slightly different physical shape. A bond between two amino acids is called a peptide bond, and a short chain of amino acids linked by peptide bonds is a polypeptide.
A protein may consist of from 50 to thousands of amino acids. The sequence of the amino acids is specific and unique for each protein, and is called its primary structure. This unique sequence, and the hydrogen bonds and disulfide bonds formed within the amino acid chain, determines how the protein will be folded to complete its synthesis. The folding may be simple, a helix (coil) or pleated sheet, called the sec-ondary structure, or a more complex folding may occur to form a globular protein, called the tertiary structure. Myoglobin, found in muscles, is a globular protein (Fig. 2–8). When complete, each protein has a characteristic three-dimensional shape, which in turn determines its function. Some proteins consist of more than one amino acid chain (quaternary struc-ture). Hemoglobin, for example, has four amino acid chains. Notice that myoglobin contains an atom of iron (a hemoglobin molecule has four iron atoms). Some proteins require a trace element such as iron or zinc to complete their structure and permit them to function properly.
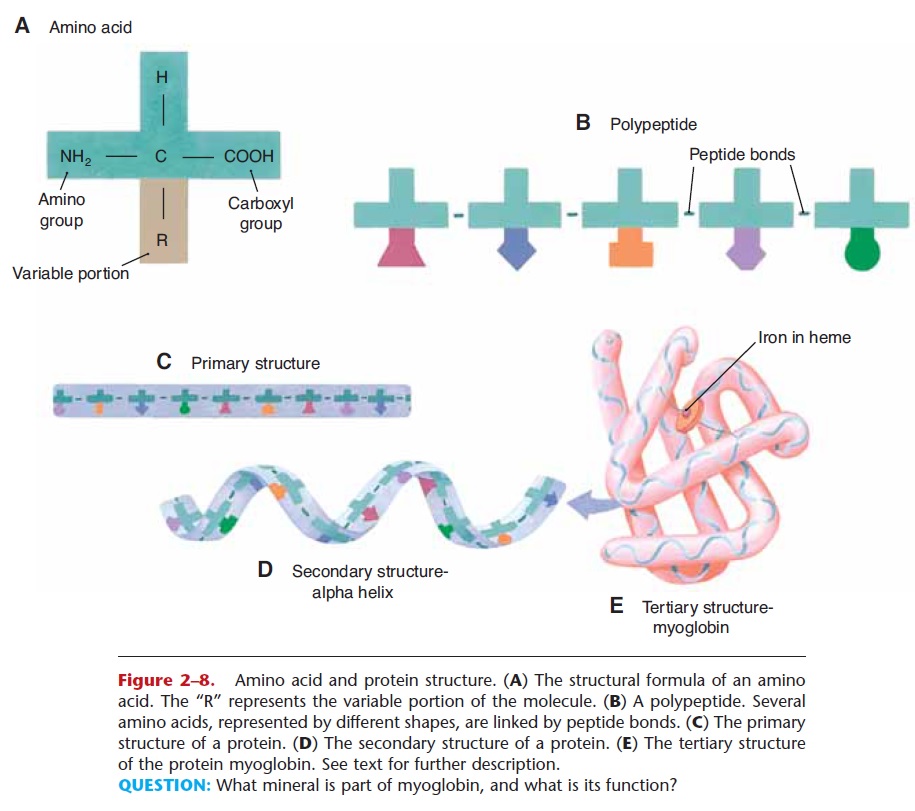
Figure 2–8. Amino acid and protein structure. (A) The structural formula of an amino acid. The “R” represents the variable portion of the molecule. (B) A polypeptide. Several amino acids, represented by different shapes, are linked by peptide bonds. (C) The primary structure of a protein. (D) The secondary structure of a protein. (E) The tertiary structure of the protein myoglobin. See text for further description.
QUESTION: What mineral is part of myoglobin, and what is its function?
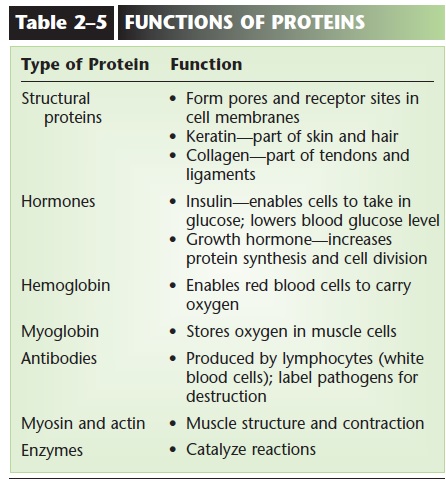
Our body proteins have many functions; some of these are listed in Table 2–5. And though we usually do not think of protein as an energy food, if our diet includes more amino acids than are necessary for our protein synthesis, those excess amino acids will be converted to simple carbohydrates or even to fat, to be stored as potential energy. One very important function of proteins will be dis-cussed further here: the role of proteins as enzymes.
Related Topics