Chapter: Essentials of Anatomy and Physiology: Some Basic Chemistry
Enzymes
Enzymes
Enzymes are catalysts, which means that they speed up chemical reactions without the need for an external source of energy such as heat. The many reactions that take place within the body are catalyzed by spe-cific enzymes; all of these reactions must take place at body temperature.
The way in which enzymes function as catalysts is called the active site theory, and is based on the shape of the enzyme and the shapes of the reacting molecules, called substrates. A simple synthesis reac-tion is depicted in Fig. 2–9A. Notice that the enzyme has a specific shape, as do the substrate molecules.
The active site of the enzyme is the part that matches the shapes of the substrates. The substrates must “fit” into the active site of the enzyme, and temporary bonds may form between the enzyme and the sub-strate. This is called the enzyme–substrate complex. In this case, two substrate molecules are thus brought close together so that chemical bonds are formed be tween them, creating a new compound. The product of the reaction, the new compound, is then released, leaving the enzyme itself unchanged and able to cat-alyze another reaction of the same type.
The reaction shown in Fig. 2–9B is a decomposition reaction. As the substrate molecule bonds to the active site of the enzyme, strain is put on its internal bonds, which break, forming two product molecules and again leaving the enzyme unchanged. Each enzyme is specific in that it will catalyze only one type of reac-tion. An enzyme that digests the protein in food, for example, has the proper shape for that reaction but cannot digest starches. For starch digestion, another enzyme with a differently shaped active site is needed. Thousands of chemical reactions take place within the body, and therefore we have thousands of enzymes, each with its own shape and active site.
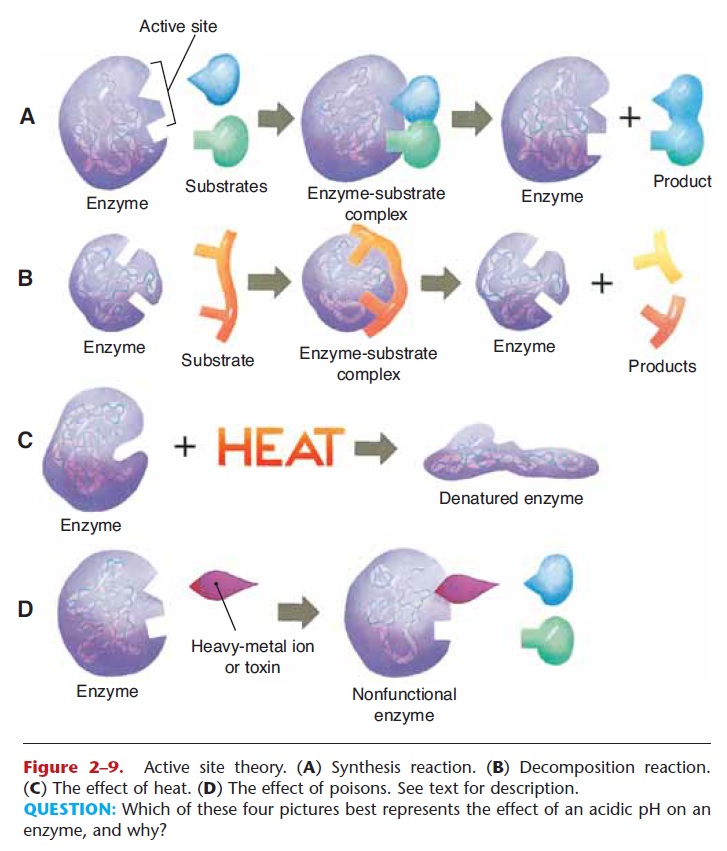
Figure 2–9. Active site theory. (A) Synthesis reaction. (B) Decomposition reaction. (C) The effect of heat. (D) The effect of poisons. See text for description.
QUESTION: Which of these four pictures best represents the effect of an acidic pH on an enzyme, and why?
The ability of enzymes to function may be limited or destroyed by changes in the intracellular or extra-cellular fluids in which they are found. Changes in pH and temperature are especially crucial. Recall that the pH of intracellular fluid is approximately 6.8, and that a decrease in pH means that more H+ ions are pres-ent. If pH decreases significantly, the excess H+ ions will react with the active sites of cellular enzymes, change their shapes, and prevent them from catalyzing reactions. This is why a state of acidosis may cause the death of cells—the cells’ enzymes are unable to func-tion properly.
With respect to temperature, most human enzymes have their optimum functioning in the normal range of body temperature: 97o to 99oF (36o to 38oC).
A temperature of 106oF, a high fever, may break the chemical bonds that maintain the shapes of enzymes (see Fig. 2–9C). If an enzyme loses its shape, it is said to be denatured, and a denatured enzyme is unable to function as a catalyst. Some human enzymes, when denatured by a high fever, may revert to their original shapes if the fever is lowered quickly. Others, however, will not. (An example of irreversible denaturation is a hard-boiled egg; the proteins in the egg white and yolk will never revert to what they were in the original egg.) A high fever may cause brain damage or death because enzymes in the brain have become permanently denatured.
You already know that metals such as lead and mercury are harmful to humans and that both may cause serious damage to the nervous system and other body tissues. These heavy metals are harmful to us because they are very reactive and block the actions of our enzymes. Fig. 2–9D depicts what happens. Notice that the heavy metal ion bonds with part of the active site of the enzyme and changes its shape. The substrate molecule cannot fit, and the enzyme is useless. Many other chemicals are poisonous to us for the very same reason: They destroy the functioning of our enzymes, and essential reactions cannot take place.
Related Topics